Hypomethylation-induced prognostic marker zinc finger DHHC-type palmitoyltransferase 12 contributes to glioblastoma progression
- PMID: 35434031
- PMCID: PMC9011314
- DOI: 10.21037/atm-22-520
Hypomethylation-induced prognostic marker zinc finger DHHC-type palmitoyltransferase 12 contributes to glioblastoma progression
Abstract
Background: Glioma is the most common intracranial primary malignancy, characterized by abnormal signal transductions caused by transcriptional and post-transcriptional regulators. Studies show the palmitoylation of oncoproteins and tumor suppressors participate in cancer progression, while studies of protein S-palmitoyltransferases in glioma are limited. A systematic analysis of zinc finger DHHC-type palmitoyltransferases (ZDHHC) in glioma is still lacking.
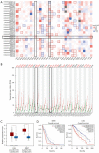
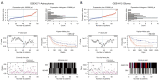
Methods: A prognostic heatmap and Kaplan-Meier overall survival plot of 24 members of the ZDHHC family in pan-cancer created. The expression and prognostic significance of ZDHHC12 was analyzed by using Gene Expression Profiling Interactive Analysis (GEPIA) and PrognoScan. DBTRG and U251 cells with silenced ZDHHC12 expression were constructed and used for cell counting kit-8 (CCK-8), Transwell assay and wound healing assay in vitro.
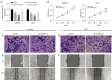
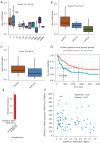
Results: Here, we first conducted expression and prognostic analyses of 24 ZDHHCs from The Cancer Genome Atlas (TCGA), the Chinese Glioma Genome Atlas (CGGA), and other glioma datasets. We found ZDHHC12 to be the only unfavorable prognostic marker in glioma. The function of ZDHHC12 in glioma was then investigated with loss-of-function strategies and in vitro cell assays. Results showed that ZDHHC12 knockdown remarkably reduced the growth, migration, and invasion capabilities in DBTRG and U251 cell lines, suggesting that ZDHHC12 may contribute to malignant behavior in glioma cells. Finally, the molecular basis for ZDHHC12 expression in glioma was analyzed, and DNA hypomethylation was found to be responsible for increased ZDHHC12 mRNA expression and related prognoses.
Conclusions: ZDHHC12 positively promoted the proliferation and migration of glioma cells. Decreased DNA methylation may lead to increased ZDHHC12 expression in gliomas. This study may deepen the understanding of glioma progression and therapeutics.
Keywords: Glioma; ZDHHC12; hypomethylation; prognosis.
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest:All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-520/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Palmitoyl transferases act as potential regulators of tumor-infiltrating immune cells and glioma progression.Mol Ther Nucleic Acids. 2022 May 4;28:716-731. doi: 10.1016/j.omtn.2022.04.030. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35664705 Free PMC article.
-
Bromodomain containing 4 transcriptionally activated Deltex E3 ubiquitin ligase 2 contributes to glioma progression and predicts an unfavorable prognosis.Ann Transl Med. 2022 Mar;10(6):313. doi: 10.21037/atm-22-555. Ann Transl Med. 2022. PMID: 35434025 Free PMC article.
-
ZDHHC15 promotes glioma malignancy and acts as a novel prognostic biomarker for patients with glioma.BMC Cancer. 2023 May 9;23(1):420. doi: 10.1186/s12885-023-10883-6. BMC Cancer. 2023. PMID: 37161425 Free PMC article.
-
Inhibition of palmitoyltransferase ZDHHC12 sensitizes ovarian cancer cells to cisplatin through ROS-mediated mechanisms.Cancer Sci. 2024 Apr;115(4):1170-1183. doi: 10.1111/cas.16085. Epub 2024 Jan 29. Cancer Sci. 2024. PMID: 38287874 Free PMC article.
-
ZDHHC12-mediated claudin-3 S-palmitoylation determines ovarian cancer progression.Acta Pharm Sin B. 2020 Aug;10(8):1426-1439. doi: 10.1016/j.apsb.2020.03.008. Epub 2020 Apr 19. Acta Pharm Sin B. 2020. PMID: 32963941 Free PMC article.
Cited by
-
Integrated analysis of genome-wide DNA methylation and cancer-associated fibroblasts identified prognostic biomarkers and immune checkpoint blockade in lower grade gliomas.Front Oncol. 2023 Jan 16;12:977251. doi: 10.3389/fonc.2022.977251. eCollection 2022. Front Oncol. 2023. PMID: 36727078 Free PMC article.
-
Diverse Roles of Protein Palmitoylation in Cancer Progression, Immunity, Stemness, and Beyond.Cells. 2023 Sep 5;12(18):2209. doi: 10.3390/cells12182209. Cells. 2023. PMID: 37759431 Free PMC article. Review.
-
Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications.MedComm (2020). 2023 May 2;4(3):e261. doi: 10.1002/mco2.261. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37143582 Free PMC article. Review.
-
Advances of Protein Palmitoylation in Tumor Cell Deaths.Cancers (Basel). 2023 Nov 21;15(23):5503. doi: 10.3390/cancers15235503. Cancers (Basel). 2023. PMID: 38067206 Free PMC article. Review.
-
Identification and prognostic biomarkers among ZDHHC4/12/18/24, and APT2 in lung adenocarcinoma.Sci Rep. 2024 Jan 4;14(1):522. doi: 10.1038/s41598-024-51182-9. Sci Rep. 2024. PMID: 38177255 Free PMC article.
References
-
- Nicholson JG, Fine HA. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov 2021;11:575-90. 10.1158/2159-8290.CD-20-1474 - DOI - PubMed
LinkOut - more resources
Full Text Sources
