Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells
- PMID: 35432205
- PMCID: PMC9009089
- DOI: 10.3389/fendo.2022.869414
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells
Abstract
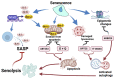
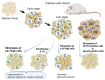
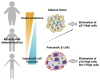
Increased insulin resistance and impaired insulin secretion are significant characteristics manifested by patients with type 2 diabetes mellitus (T2DM). The degree and extent of these two features in T2DM vary among races and individuals. Insulin resistance is accelerated by obesity and is accompanied by accumulation of dysfunctional adipose tissues. In addition, dysfunction of pancreatic β-cells impairs insulin secretion. T2DM is significantly affected by aging, as the β-cell mass diminishes with age. Moreover, both obesity and hyperglycemia-related metabolic changes in developing diabetes are associated with accumulation of senescent cells in multiple organs, that is, organismal aging. Cellular senescence is defined as a state of irreversible cell cycle arrest with concomitant functional decline. It is caused by telomere shortening or senescence-inducing stress. Senescent cells secrete proinflammatory cytokines and chemokines, which is designated as the senescence-associated secretory phenotype (SASP), and this has a negative impact on adipose tissues and pancreatic β-cells. Recent advances in aging research have suggested that senolysis, the removal of senescent cells, can be a promising therapeutic approach to prevent or improve aging-related diseases, including diabetes. The attenuation of a SASP may be beneficial, although the pathophysiological involvement of cellular senescence in diabetes is not fully understood. In the clinical application of senotherapy, tissue-context-dependent senescent cells are increasingly being recognized as an issue to be solved. Recent studies have observed highly heterogenic and complex senescent cell populations that serve distinct roles among tissues, various stages of disease, and different ages. For example, in high-fat-diet induced diabetes with obesity, mouse adipose tissues display accumulation of p21Cip1-highly-expressing (p21high) cells in the early stage, followed by increases in both p21high and p16INK4a-highly-expressing (p16high) cells in the late stage. Interestingly, elimination of p21high cells in visceral adipose tissue can prevent or improve insulin resistance in mice with obesity, while p16high cell clearance is less effective in alleviating insulin resistance. Importantly, in immune-deficient mice transplanted with fat from obese patients, dasatinib plus quercetin, a senolytic cocktail that reduces the number of both p21high and p16high cells, improves both glucose tolerance and insulin resistance. On the other hand, in pancreatic β cells, p16high cells become increasingly predominant with age and development of diabetes. Consistently, elimination of p16high cells in mice improves both glucose tolerance and glucose-induced insulin secretion. Moreover, a senolytic compound, the anti-Bcl-2 inhibitor ABT263 reduces p16INK4a expression in islets and restores glucose tolerance in mice when combined with insulin receptor antagonist S961 treatment. In addition, efficacy of senotherapy in targeting mouse pancreatic β cells has been validated not only in T2DM, but also in type 1 diabetes mellitus. Indeed, in non-obese diabetic mice, treatment with anti-Bcl-2 inhibitors, such as ABT199, eliminates senescent pancreatic β cells, resulting in prevention of diabetes mellitus. These findings clearly indicate that features of diabetes are partly determined by which or where senescent cells reside in vivo, as adipose tissues and pancreatic β cells are responsible for insulin resistance and insulin secretion, respectively. In this review, we summarize recent advances in understanding cellular senescence in adipose tissues and pancreatic β cells in diabetes. We review the different potential molecular targets and distinctive senotherapeutic strategies in adipose tissues and pancreatic β cells. We propose the novel concept of a dual-target tailored approach in senotherapy against diabetes.
Keywords: adipose tissue; aging; cellular senescence; diabetes mellitus; obesity; pancreatic β cell; senolysis; tailored senotherapy.
Copyright © 2022 Murakami, Inagaki and Kondoh.
Conflict of interest statement
NI received clinical commissioned/joint research grants from Daiichi Sankyo, Terumo, and Drawbridge Inc., speaker honoraria from Kowa, MSD, Astellas Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Takeda, Eli Lilly Japan, Sumitomo Dainippon Pharma, and Mitsubishi Tanabe Pharma, scholarship grants from Kissei Pharmaceutical, Sanofi, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Takeda, Japan Tobacco, Kyowa Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, MSD, Eli Lilly Japan, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Novartis Pharma, Teijin Pharma, and Life Scan Japan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes.Cell Metab. 2019 Jul 2;30(1):129-142.e4. doi: 10.1016/j.cmet.2019.05.006. Epub 2019 May 30. Cell Metab. 2019. PMID: 31155496 Free PMC article.
-
Targeting senescent cells alleviates obesity-induced metabolic dysfunction.Aging Cell. 2019 Jun;18(3):e12950. doi: 10.1111/acel.12950. Epub 2019 Mar 25. Aging Cell. 2019. PMID: 30907060 Free PMC article.
-
Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity.Aging Cell. 2022 May;21(5):e13602. doi: 10.1111/acel.13602. Epub 2022 Apr 1. Aging Cell. 2022. PMID: 35363946 Free PMC article.
-
Role of Cellular Senescence in Type II Diabetes.Endocrinology. 2021 Oct 1;162(10):bqab136. doi: 10.1210/endocr/bqab136. Endocrinology. 2021. PMID: 34363464 Free PMC article. Review.
-
Cellular senescence and its role in white adipose tissue.Int J Obes (Lond). 2021 May;45(5):934-943. doi: 10.1038/s41366-021-00757-x. Epub 2021 Jan 28. Int J Obes (Lond). 2021. PMID: 33510393 Review.
Cited by
-
Eternal Youth: A Comprehensive Exploration of Gene, Cellular, and Pharmacological Anti-Aging Strategies.Int J Mol Sci. 2024 Jan 4;25(1):643. doi: 10.3390/ijms25010643. Int J Mol Sci. 2024. PMID: 38203812 Free PMC article. Review.
-
Cutaneous Redox Senescence.Biomedicines. 2024 Feb 1;12(2):348. doi: 10.3390/biomedicines12020348. Biomedicines. 2024. PMID: 38397950 Free PMC article. Review.
-
Irisin suppresses pancreatic β cell pyroptosis in T2DM by inhibiting the NLRP3-GSDMD pathway and activating the Nrf2-TrX/TXNIP signaling axis.Diabetol Metab Syndr. 2023 Nov 22;15(1):239. doi: 10.1186/s13098-023-01216-5. Diabetol Metab Syndr. 2023. PMID: 37993958 Free PMC article.
-
The emerging roles and mechanisms of exosomal non-coding RNAs in the mutual regulation between adipose tissue and other related tissues in obesity and metabolic diseases.Front Endocrinol (Lausanne). 2022 Aug 18;13:975334. doi: 10.3389/fendo.2022.975334. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060952 Free PMC article. Review.
-
High-fat diet impairs glucose homeostasis by increased p16 beta-cell expression and alters glucose homeostasis of the progeny in a parental-sex dependent manner.Front Endocrinol (Lausanne). 2023 Oct 9;14:1246194. doi: 10.3389/fendo.2023.1246194. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37876538 Free PMC article.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; (2021). Available at: http://www.idf.org/diabetesatlas.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

