Facial Stimulation Induces Long-Term Potentiation of Mossy Fiber-Granule Cell Synaptic Transmission via GluN2A-Containing N-Methyl-D-Aspartate Receptor/Nitric Oxide Cascade in the Mouse Cerebellum
- PMID: 35431815
- PMCID: PMC9005984
- DOI: 10.3389/fncel.2022.863342
Facial Stimulation Induces Long-Term Potentiation of Mossy Fiber-Granule Cell Synaptic Transmission via GluN2A-Containing N-Methyl-D-Aspartate Receptor/Nitric Oxide Cascade in the Mouse Cerebellum
Abstract
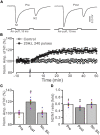
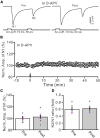
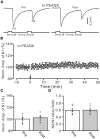
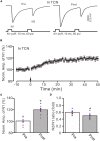
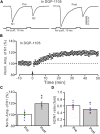
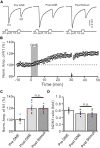
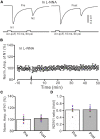
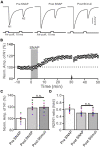
Long-term synaptic plasticity in the cerebellar cortex is a possible mechanism for motor learning. Previous studies have demonstrated the induction of mossy fiber-granule cell (MF-GrC) synaptic plasticity under in vitro and in vivo conditions, but the mechanisms underlying sensory stimulation-evoked long-term synaptic plasticity of MF-GrC in living animals are unclear. In this study, we investigated the mechanism of long-term potentiation (LTP) of MF-GrC synaptic transmission in the cerebellum induced by train of facial stimulation at 20 Hz in urethane-anesthetized mice using electrophysiological recording, immunohistochemistry techniques, and pharmacological methods. Blockade of GABAA receptor activity and repetitive facial stimulation at 20 Hz (240 pulses) induced an LTP of MF-GrC synapses in the mouse cerebellar cortical folium Crus II, accompanied with a decrease in paired-pulse ratio (N2/N1). The facial stimulation-induced MF-GrC LTP was abolished by either an N-methyl-D-aspartate (NMDA) receptor blocker, i.e., D-APV, or a specific GluNR2A subunit-containing NMDA receptor antagonist, PEAQX, but was not prevented by selective GluNR2B or GluNR2C/D subunit-containing NMDA receptor blockers. Application of GNE-0723, a selective and brain-penetrant-positive allosteric modulator of GluN2A subunit-containing NMDA receptors, produced an LTP of N1, accompanied with a decrease in N2/N1 ratio, and occluded the 20-Hz facial stimulation-induced MF-GrC LTP. Inhibition of nitric oxide synthesis (NOS) prevented the facial stimulation-induced MF-GrC LTP, while activation of NOS produced an LTP of N1, with a decrease in N2/N1 ratio, and occluded the 20-Hz facial stimulation-induced MF-GrC LTP. In addition, GluN2A-containing NMDA receptor immunoreactivity was observed in the mouse cerebellar granular layer. These results indicate that facial stimulation at 20 Hz induced LTP of MF-GrC synaptic transmission via the GluN2A-containing NMDA receptor/nitric oxide cascade in mice. The results suggest that the sensory stimulation-evoked LTP of MF-GrC synaptic transmission in the granular layer may play a critical role in cerebellar adaptation to native mossy fiber excitatory inputs and motor learning behavior in living animals.
Keywords: NMDA receptor; cerebellum; in vivo electrophysiological recording; mossy fiber-granule cell synaptic transmission; nitric oxide; plasticity; sensory stimulation.
Copyright © 2022 Lu, Wan, Liu, Jin, Chu, Bing and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Nicotine Facilitates Facial Stimulation-Evoked Mossy Fiber-Granule Cell Long-Term Potentiation in vivo in Mice.Front Cell Neurosci. 2022 Jul 4;16:905724. doi: 10.3389/fncel.2022.905724. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35860314 Free PMC article.
-
Gestational valproic acid exposure enhances facial stimulation-evoked cerebellar mossy fiber-granule cell transmission via GluN2A subunit-containing NMDA receptor in offspring mice.Transl Psychiatry. 2024 Jul 3;14(1):272. doi: 10.1038/s41398-024-02990-0. Transl Psychiatry. 2024. PMID: 38961057 Free PMC article.
-
NMDARs contribute to the facial stimuli-evoked mossy fiber-granule cell synaptic transmission in vivo in mice.Neurosci Lett. 2020 Sep 25;736:135285. doi: 10.1016/j.neulet.2020.135285. Epub 2020 Aug 2. Neurosci Lett. 2020. PMID: 32755629
-
Long-term potentiation of synaptic transmission at the mossy fiber-granule cell relay of cerebellum.Prog Brain Res. 2005;148:69-80. doi: 10.1016/S0079-6123(04)48007-8. Prog Brain Res. 2005. PMID: 15661182 Review.
-
Synaptic plasticity at the cerebellum input stage: mechanisms and functional implications.Arch Ital Biol. 2005 May;143(2):143-56. Arch Ital Biol. 2005. PMID: 16106995 Review.
Cited by
-
Nicotine Facilitates Facial Stimulation-Evoked Mossy Fiber-Granule Cell Long-Term Potentiation in vivo in Mice.Front Cell Neurosci. 2022 Jul 4;16:905724. doi: 10.3389/fncel.2022.905724. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35860314 Free PMC article.
-
Understanding Cerebellar Input Stage through Computational and Plasticity Rules.Biology (Basel). 2024 Jun 1;13(6):403. doi: 10.3390/biology13060403. Biology (Basel). 2024. PMID: 38927283 Free PMC article. Review.
-
Gestational valproic acid exposure enhances facial stimulation-evoked cerebellar mossy fiber-granule cell transmission via GluN2A subunit-containing NMDA receptor in offspring mice.Transl Psychiatry. 2024 Jul 3;14(1):272. doi: 10.1038/s41398-024-02990-0. Transl Psychiatry. 2024. PMID: 38961057 Free PMC article.
References
-
- Bhattacharya S., Khatri A., Swanger S. A., DiRaddo J. O., Yi F., Hansen K. B., et al. (2018). Triheteromeric GluN1/GluN2A/GluN2C NMDARs with unique single-channel properties are the dominant receptor population in cerebellar granule cells. Neuron 99 315–328. 10.1016/j.neuron.2018.06.010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

