Mubritinib enhanced the inhibiting function of cisplatin in lung cancer by interfering with mitochondrial function
- PMID: 35429141
- PMCID: PMC9108040
- DOI: 10.1111/1759-7714.14425
Mubritinib enhanced the inhibiting function of cisplatin in lung cancer by interfering with mitochondrial function
Abstract
Background: Lung cancer is one of the most lethal cancers worldwide. Cisplatin, a widely used anti-lung cancer drug, has been limited in clinical application due to its drug resistance. Medicines targeting mitochondrial electron transport chain (ETC) complexes may be effective candidates for cisplatin-based chemotherapy.
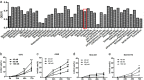
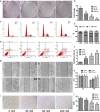
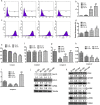
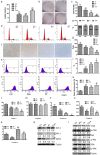
Methods: In this study, the small molecule drug library from Food and Drug Administration FDA was used to screen for medicines targeting ETC. MTT and colony formation assays were used to investigate cell proliferation. Flow cytometry was employed to analyze cell cycle, apoptosis, reactive oxygen species (ROS), and mitochondrial membrane potential. Wound scratch and transwell assays were used to detect migration and invasion abilities. The activities of the ETC complex were tested using kits. Western blot analysis was used to investigate the expressions of related proteins. A mouse xenograft model was constructed to verify the antitumor effect in vivo.
Results: The results showed that mubritinib can reduce the activation of the PI3K/mTOR signal pathway, disrupt mitochondrial function, significantly increase ROS levels and induce oxidative stress, and ultimately exert its antitumor effect against non-small cell lung cancer (NSCLC) both in vivo and in vitro. In addition, the combination of cisplatin and mubritinib can improve the tumor-suppressive effect of cisplatin.
Conclusion: Mubritinib can upregulate intracellular ROS concentration and cell apoptosis, inhibit the PI3K signaling pathway and interfere with the function of mitochondria, thus reducing cell proliferation and increasing ROS induced apoptosis by reducing the activation of Nrf2 by PI3K.
Keywords: PI3K/mTOR pathway; ROS; lung cancer; mitochondrial electron transport chain; mubritinib.
© 2022 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Silencing long non-coding RNA ROR improves sensitivity of non-small-cell lung cancer to cisplatin resistance by inhibiting PI3K/Akt/mTOR signaling pathway.Tumour Biol. 2017 May;39(5):1010428317697568. doi: 10.1177/1010428317697568. Tumour Biol. 2017. PMID: 28459375
-
Antitumor effects of flavokawain-B flavonoid in gemcitabine-resistant lung cancer cells are mediated via mitochondrial-mediated apoptosis, ROS production, cell migration and cell invasion inhibition and blocking of PI3K/AKT Signaling pathway.J BUON. 2020 Jan-Feb;25(1):262-267. J BUON. 2020. PMID: 32277640
-
Investigation on the mechanism of the combination of eremias multiocellata and cisplatin in reducing chemoresistance of gastric cancer based on in vitro and in vivo experiments.Aging (Albany NY). 2024 Feb 9;16(4):3386-3403. doi: 10.18632/aging.205540. Epub 2024 Feb 9. Aging (Albany NY). 2024. PMID: 38345573 Free PMC article.
-
Fuzheng Jiedu Decoction Induces Apoptosis and Enhances Cisplatin Efficacy in Ovarian Cancer Cells In Vitro and In Vivo through Inhibiting the PI3K/AKT/mTOR/NF-κB Signaling Pathway.Biomed Res Int. 2022 Mar 2;2022:5739909. doi: 10.1155/2022/5739909. eCollection 2022. Biomed Res Int. 2022. PMID: 35281608 Free PMC article.
-
Molecular mechanism of ferulic acid and its derivatives in tumor progression.Pharmacol Rep. 2023 Aug;75(4):891-906. doi: 10.1007/s43440-023-00494-0. Epub 2023 May 18. Pharmacol Rep. 2023. PMID: 37202657 Free PMC article. Review.
Cited by
-
Stellera chamaejasme L. extracts in the treatment of glioblastoma cell lines: Biological verification based on a network pharmacology approach.Front Oncol. 2022 Aug 17;12:962970. doi: 10.3389/fonc.2022.962970. eCollection 2022. Front Oncol. 2022. PMID: 36059675 Free PMC article.
-
Methylene Blue Induces Antioxidant Defense and Reparation of Mitochondrial DNA in a Nrf2-Dependent Manner during Cisplatin-Induced Renal Toxicity.Int J Mol Sci. 2023 Mar 24;24(7):6118. doi: 10.3390/ijms24076118. Int J Mol Sci. 2023. PMID: 37047089 Free PMC article.
-
The HIF-1α and mTOR Pathways Amplify Heterotopic Ossification.Biomolecules. 2024 Jan 24;14(2):147. doi: 10.3390/biom14020147. Biomolecules. 2024. PMID: 38397384 Free PMC article. Review.
-
βIII-tubulin suppression enhances the activity of Amuvatinib to inhibit cell proliferation in c-Met positive non-small cell lung cancer cells.Cancer Med. 2023 Feb;12(4):4455-4471. doi: 10.1002/cam4.5128. Epub 2022 Aug 10. Cancer Med. 2023. PMID: 35946957 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

