Proliferative and anti-apoptotic fractions in maturing hematopoietic cell lineages and their role in homeostasis of normal bone marrow
- PMID: 35429122
- PMCID: PMC9540078
- DOI: 10.1002/cyto.a.24558
Proliferative and anti-apoptotic fractions in maturing hematopoietic cell lineages and their role in homeostasis of normal bone marrow
Abstract
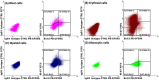
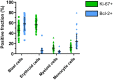
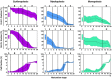
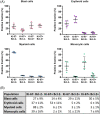
Recent developments in clinical flow cytometry allow the simultaneous assessment of proliferative and anti-apoptotic activity in the different hematopoietic cell lineages and during their maturation process. This can further advance the flow cytometric diagnosis of myeloid malignancies. In this study we established indicative reference values for the Ki-67 proliferation index and Bcl-2 anti-apoptotic index in blast cells, as well as maturing erythroid, myeloid, and monocytic cells from normal bone marrow (BM). Furthermore, the cell fractions co-expressing both proliferation and anti-apoptotic markers were quantified. Fifty BM aspirates from femoral heads of patients undergoing hip replacement were included in this study. Ten-color/twelve-parameter flow cytometry in combination with a software-based maturation tool was used for immunophenotypic analysis of Ki-67 and Bcl-2 positive fractions during the erythro-, myelo-, and monopoiesis. Indicative reference values for the Ki-67 and Bcl-2 positive fractions were established for different relevant hematopoietic cell populations in healthy BM. Ki-67 and Bcl-2 were equally expressed in the total CD34 positive blast cell compartment and 30% of Ki-67 positive blast cells also showed Bcl-2 positivity. The Ki-67 and Bcl-2 positive fractions were highest in the more immature erythroid, myeloid and monocytic cells. Both fractions then gradually declined during the subsequent maturation phases of these cell lineages. We present a novel application of an earlier developed assay that allows the simultaneous determination of the Ki-67 proliferative and Bcl-2 anti-apoptotic indices in maturing hematopoietic cell populations of the BM. Their differential expression levels during the maturation process were in accordance with the demand and lifespan of these cell populations. The indicative reference values established in this study can act as a baseline for further cell biological and biomedical studies involving hematological malignancies.
Keywords: Bcl-2; Ki-67; anti-apoptosis; hematopoietic cell lineages; maturation; multiparameter flow cytometry; normal bone marrow; proliferation.
© 2022 The Authors. Cytometry Part A published by Wiley Periodicals LLC on behalf of International Society for Advancement of Cytometry.
Conflict of interest statement
F.C.S.R. is CSO and QA manager at Nordic‐MUbio, Susteren, The Netherlands.
Figures


Similar articles
-
Determination of the proliferative fractions in differentiating hematopoietic cell lineages of normal bone marrow.Cytometry A. 2018 Nov;93(11):1097-1105. doi: 10.1002/cyto.a.23564. Epub 2018 Sep 3. Cytometry A. 2018. PMID: 30176186
-
Impact of the gating strategy for Ki-67 and Bcl-2 on the determination of proliferation and anti-apoptosis data by flow cytometry in non-malignant bone marrow aspirates and aspirates from patients with myeloid malignancies.Data Brief. 2023 Jun 9;49:109284. doi: 10.1016/j.dib.2023.109284. eCollection 2023 Aug. Data Brief. 2023. PMID: 37409174 Free PMC article.
-
MDS and AML show elevated fractions of CD34-positive blast cell populations with a high anti-apoptotic versus proliferation ratio.Leuk Res. 2024 Jul;142:107520. doi: 10.1016/j.leukres.2024.107520. Epub 2024 May 15. Leuk Res. 2024. PMID: 38776565
-
The potential of proliferative and apoptotic parameters in clinical flow cytometry of myeloid malignancies.Blood Adv. 2021 Apr 13;5(7):2040-2052. doi: 10.1182/bloodadvances.2020004094. Blood Adv. 2021. PMID: 33847740 Free PMC article. Review.
-
Bcl-2 expression in acute myeloblastic leukaemia: relationship with autonomous growth and CD34 antigen expression.Leuk Lymphoma. 1997 Jan;24(3-4):221-8. doi: 10.3109/10428199709039010. Leuk Lymphoma. 1997. PMID: 9156652 Review.
Cited by
-
The Proliferation Index of Erythroid Cells Predicts the Development of Transfusion-dependence in Myelodysplastic Syndrome Patients With Mildly Reduced Hemoglobin Levels at Initial Diagnosis.Hemasphere. 2022 Nov 9;6(12):e804. doi: 10.1097/HS9.0000000000000804. eCollection 2022 Dec. Hemasphere. 2022. PMID: 36382052 Free PMC article. No abstract available.
References
-
- Alenzi FQB. Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci. 2004;61:99–102. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Stuart‐Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta‐analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–34. - PubMed
-
- Broyde A, Boycov O, Strenov Y, Okon E, Shpilberg O, Bairey O. Role and prognostic significance of the Ki‐67 index in non‐Hodgkin's lymphoma. Am J Hematol. 2009;84:338–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

