Emerging therapies for glioblastoma: current state and future directions
- PMID: 35428347
- PMCID: PMC9013078
- DOI: 10.1186/s13046-022-02349-7
Emerging therapies for glioblastoma: current state and future directions
Abstract
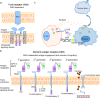
Glioblastoma (GBM) is the most common high-grade primary malignant brain tumor with an extremely poor prognosis. Given the poor survival with currently approved treatments for GBM, new therapeutic strategies are urgently needed. Advances in decades of investment in basic science of glioblastoma are rapidly translated into innovative clinical trials, utilizing improved genetic and epigenetic profiling of glioblastoma as well as the brain microenvironment and immune system interactions. Following these encouraging findings, immunotherapy including immune checkpoint blockade, chimeric antigen receptor T (CAR T) cell therapy, oncolytic virotherapy, and vaccine therapy have offered new hope for improving GBM outcomes; ongoing studies are using combinatorial therapies with the aim of minimizing adverse side-effects and augmenting antitumor immune responses. In addition, techniques to overcome the blood-brain barrier (BBB) for targeted delivery are being tested in clinical trials in patients with recurrent GBM. Here, we set forth the rationales for these promising therapies in treating GBM, review the potential novel agents, the current status of preclinical and clinical trials, and discuss the challenges and future perspectives in glioblastoma immuno-oncology.
Keywords: CAR T, Oncolytic virotherapy; Focused ultrasound; Glioblastoma; Immune checkpoint blockade; Immunotherapy; Vaccine.
© 2022. The Author(s).
Conflict of interest statement
The author declare that there is no conflict of interests regarding the publication of this paper.
Figures

Similar articles
-
Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds.Int Rev Immunol. 2022;41(6):582-605. doi: 10.1080/08830185.2022.2101647. Epub 2022 Aug 8. Int Rev Immunol. 2022. PMID: 35938932 Review.
-
Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme.Cancer Med. 2021 Aug;10(15):5019-5030. doi: 10.1002/cam4.4064. Epub 2021 Jun 19. Cancer Med. 2021. PMID: 34145792 Free PMC article. Review.
-
Glioblastoma Immunotherapy: A Systematic Review of the Present Strategies and Prospects for Advancements.Int J Mol Sci. 2023 Oct 10;24(20):15037. doi: 10.3390/ijms242015037. Int J Mol Sci. 2023. PMID: 37894718 Free PMC article. Review.
-
Emerging immunotherapies for glioblastoma.Expert Opin Emerg Drugs. 2016 Jun;21(2):133-45. doi: 10.1080/14728214.2016.1186643. Expert Opin Emerg Drugs. 2016. PMID: 27223671 Free PMC article. Review.
-
From promise to progress: the dynamic landscape of glioblastoma immunotherapy.Drug Discov Today. 2024 Nov;29(11):104188. doi: 10.1016/j.drudis.2024.104188. Epub 2024 Sep 20. Drug Discov Today. 2024. PMID: 39307298 Review.
Cited by
-
Exosomal DNA: Role in Reflecting Tumor Genetic Heterogeneity, Diagnosis, and Disease Monitoring.Cancers (Basel). 2023 Dec 21;16(1):57. doi: 10.3390/cancers16010057. Cancers (Basel). 2023. PMID: 38201485 Free PMC article. Review.
-
Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro.Nanomaterials (Basel). 2023 Dec 24;14(1):55. doi: 10.3390/nano14010055. Nanomaterials (Basel). 2023. PMID: 38202510 Free PMC article.
-
Small Molecule Immunomodulators as Next-Generation Therapeutics for Glioblastoma.Cancers (Basel). 2024 Jan 19;16(2):435. doi: 10.3390/cancers16020435. Cancers (Basel). 2024. PMID: 38275876 Free PMC article. Review.
-
The Role of Transglutaminase 2 in Cancer: An Update.Int J Mol Sci. 2024 Feb 28;25(5):2797. doi: 10.3390/ijms25052797. Int J Mol Sci. 2024. PMID: 38474044 Free PMC article. Review.
-
FOSL1's Oncogene Roles in Glioma/Glioma Stem Cells and Tumorigenesis: A Comprehensive Review.Int J Mol Sci. 2024 May 14;25(10):5362. doi: 10.3390/ijms25105362. Int J Mol Sci. 2024. PMID: 38791400 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

