Design, synthesis and evaluation of 3-phenoxypyrazine-2-carboxamide derivatives as potent TGR5 agonists
- PMID: 35425398
- PMCID: PMC8979340
- DOI: 10.1039/d1ra08867j
Design, synthesis and evaluation of 3-phenoxypyrazine-2-carboxamide derivatives as potent TGR5 agonists
Abstract
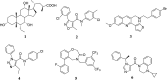
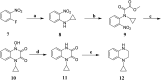
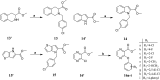
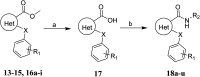
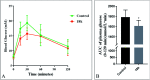
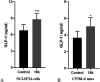
TGR5 is emerging as an important and promising target for the treatment of non-alcoholic steatohepatitis, type 2 diabetes mellitus (T2DM), and obesity. A series of novel 3-phenoxypyrazine-2-carboxamide derivatives were designed, synthesized and evaluated in vitro and in vivo. The most potent compounds 18g and 18k exhibited excellent hTGR5 agonist activity, which was superior to those of the reference drug INT-777. In addition, compound 18k could significantly reduce blood glucose levels in C57 BL/6 mice and stimulate GLP-1 secretion in NCI-H716 cells and C57 BL/6 mice.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Design, synthesis and evaluation of 1-benzyl-1H-imidazole-5-carboxamide derivatives as potent TGR5 agonists.Bioorg Med Chem. 2021 Feb 15;32:115972. doi: 10.1016/j.bmc.2020.115972. Epub 2020 Dec 27. Bioorg Med Chem. 2021. PMID: 33440321
-
Discovery of Orally Efficacious Tetrahydrobenzimidazoles as TGR5 Agonists for Type 2 Diabetes.ACS Med Chem Lett. 2017 Apr 21;8(5):560-565. doi: 10.1021/acsmedchemlett.7b00116. eCollection 2017 May 11. ACS Med Chem Lett. 2017. PMID: 28523111 Free PMC article.
-
TGR5 agonist ameliorates insulin resistance in the skeletal muscles and improves glucose homeostasis in diabetic mice.Metabolism. 2019 Oct;99:45-56. doi: 10.1016/j.metabol.2019.07.003. Epub 2019 Jul 8. Metabolism. 2019. PMID: 31295453
-
Clinical relevance of the bile acid receptor TGR5 in metabolism.Lancet Diabetes Endocrinol. 2017 Mar;5(3):224-233. doi: 10.1016/S2213-8587(16)30155-3. Epub 2016 Sep 14. Lancet Diabetes Endocrinol. 2017. PMID: 27639537 Review.
-
Potential New Approaches to Modifying Intestinal GLP-1 Secretion in Patients with Type 2 Diabetes Mellitus : Focus on Bile Acid Sequestrants.Clin Drug Investig. 2012 Jan;32(1):1-14. doi: 10.2165/11595370-000000000-00000. Clin Drug Investig. 2012. PMID: 27933595 Review.
Cited by
-
Mechanism of action of the bile acid receptor TGR5 in obesity.Acta Pharm Sin B. 2024 Feb;14(2):468-491. doi: 10.1016/j.apsb.2023.11.011. Epub 2023 Nov 10. Acta Pharm Sin B. 2024. PMID: 38322325 Free PMC article. Review.
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct Target Ther. 2024. PMID: 38664391 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

