CENP-N promotes the compaction of centromeric chromatin
- PMID: 35422519
- PMCID: PMC9010303
- DOI: 10.1038/s41594-022-00758-y
CENP-N promotes the compaction of centromeric chromatin
Abstract
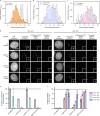
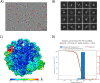
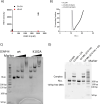
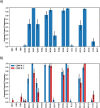
The histone variant CENP-A is the epigenetic determinant for the centromere, where it is interspersed with canonical H3 to form a specialized chromatin structure that nucleates the kinetochore. How nucleosomes at the centromere arrange into higher order structures is unknown. Here we demonstrate that the human CENP-A-interacting protein CENP-N promotes the stacking of CENP-A-containing mononucleosomes and nucleosomal arrays through a previously undefined interaction between the α6 helix of CENP-N with the DNA of a neighboring nucleosome. We describe the cryo-EM structures and biophysical characterization of such CENP-N-mediated nucleosome stacks and nucleosomal arrays and demonstrate that this interaction is responsible for the formation of densely packed chromatin at the centromere in the cell. Our results provide first evidence that CENP-A, together with CENP-N, promotes specific chromatin higher order structure at the centromere.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures









Similar articles
-
Structural mechanisms of centromeric nucleosome recognition by the kinetochore protein CENP-N.Science. 2018 Jan 19;359(6373):339-343. doi: 10.1126/science.aar2781. Epub 2017 Dec 21. Science. 2018. PMID: 29269420 Free PMC article.
-
Cryo-EM Structures of Centromeric Tri-nucleosomes Containing a Central CENP-A Nucleosome.Structure. 2020 Jan 7;28(1):44-53.e4. doi: 10.1016/j.str.2019.10.016. Epub 2019 Nov 8. Structure. 2020. PMID: 31711756
-
Direct observation of coordinated assembly of individual native centromeric nucleosomes.EMBO J. 2023 Sep 4;42(17):e114534. doi: 10.15252/embj.2023114534. Epub 2023 Jul 20. EMBO J. 2023. PMID: 37469281 Free PMC article.
-
The Elusive Structure of Centro-Chromatin: Molecular Order or Dynamic Heterogenetity?J Mol Biol. 2021 Mar 19;433(6):166676. doi: 10.1016/j.jmb.2020.10.010. Epub 2020 Oct 14. J Mol Biol. 2021. PMID: 33065112 Review.
-
The CENP-A nucleosome: a dynamic structure and role at the centromere.Chromosome Res. 2012 Jul;20(5):465-79. doi: 10.1007/s10577-012-9301-4. Chromosome Res. 2012. PMID: 22825424 Free PMC article. Review.
Cited by
-
Structure of the human inner kinetochore CCAN complex and its significance for human centromere organization.Mol Cell. 2022 Jun 2;82(11):2113-2131.e8. doi: 10.1016/j.molcel.2022.04.027. Epub 2022 May 6. Mol Cell. 2022. PMID: 35525244 Free PMC article.
-
The Four Causes: The Functional Architecture of Centromeres and Kinetochores.Annu Rev Genet. 2022 Nov 30;56:279-314. doi: 10.1146/annurev-genet-072820-034559. Epub 2022 Sep 2. Annu Rev Genet. 2022. PMID: 36055650 Free PMC article. Review.
-
Satellite DNAs in Health and Disease.Genes (Basel). 2022 Jun 26;13(7):1154. doi: 10.3390/genes13071154. Genes (Basel). 2022. PMID: 35885937 Free PMC article. Review.
-
Vertebrate centromeres in mitosis are functionally bipartite structures stabilized by cohesin.Cell. 2024 Jun 6;187(12):3006-3023.e26. doi: 10.1016/j.cell.2024.04.014. Epub 2024 May 13. Cell. 2024. PMID: 38744280 Free PMC article.
-
Vertebrate centromere architecture: from chromatin threads to functional structures.Chromosoma. 2024 Jul;133(3):169-181. doi: 10.1007/s00412-024-00823-z. Epub 2024 Jun 10. Chromosoma. 2024. PMID: 38856923 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

