Muscle dysfunction in the long coronavirus disease 2019 syndrome: Pathogenesis and clinical approach
- PMID: 35416359
- PMCID: PMC9111061
- DOI: 10.1002/rmv.2355
Muscle dysfunction in the long coronavirus disease 2019 syndrome: Pathogenesis and clinical approach
Abstract
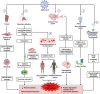
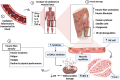
In long coronavirus disease 2019 (long COVID-19), involvement of the musculoskeletal system is characterised by the persistence or appearance of symptoms such as fatigue, muscle weakness, myalgia, and decline in physical and functional performance, even at 4 weeks after the onset of acute symptoms of COVID-19. Muscle injury biomarkers are altered during the acute phase of the disease. The cellular damage and hyperinflammatory state induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may contribute to the persistence of symptoms, hypoxaemia, mitochondrial damage, and dysregulation of the renin-angiotensin system. In addition, the occurrence of cerebrovascular diseases, involvement of the peripheral nervous system, and harmful effects of hospitalisation, such as the use of drugs, immobility, and weakness acquired in the intensive care unit, all aggravate muscle damage. Here, we review the multifactorial mechanisms of muscle tissue injury, aggravating conditions, and associated sequelae in long COVID-19.
Keywords: long COVID-19; muscle; muscle dysfunction; muscle sequelae.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest declared.
Figures

Similar articles
-
Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19.Eur J Phys Rehabil Med. 2021 Aug;57(4):653-662. doi: 10.23736/S1973-9087.20.06563-6. Epub 2021 Jan 4. Eur J Phys Rehabil Med. 2021. PMID: 33393277
-
Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19.J Cachexia Sarcopenia Muscle. 2022 Feb;13(1):11-22. doi: 10.1002/jcsm.12896. Epub 2022 Jan 7. J Cachexia Sarcopenia Muscle. 2022. PMID: 34997689 Free PMC article. Review.
-
Persistent post-COVID-19 neuromuscular symptoms.Muscle Nerve. 2023 Oct;68(4):350-355. doi: 10.1002/mus.27940. Epub 2023 Jul 19. Muscle Nerve. 2023. PMID: 37466117 Review.
-
Musculoskeletal Consequences of COVID-19.J Bone Joint Surg Am. 2020 Jul 15;102(14):1197-1204. doi: 10.2106/JBJS.20.00847. J Bone Joint Surg Am. 2020. PMID: 32675661 Free PMC article.
-
What Do We Need to Know About Musculoskeletal Manifestations of COVID-19?: A Systematic Review.JBJS Rev. 2022 Jun 3;10(6). doi: 10.2106/JBJS.RVW.22.00013. eCollection 2022 Jun 1. JBJS Rev. 2022. PMID: 35658089
Cited by
-
Gastrocnemius electrical stimulation increases ankle dorsiflexion strength in patients with post-acute sequelae of SARS-COV-2 (PASC): a double-blind randomized controlled trial.Sci Rep. 2024 Aug 2;14(1):17939. doi: 10.1038/s41598-024-68100-8. Sci Rep. 2024. PMID: 39095520 Free PMC article. Clinical Trial.
-
Long COVID as a Tauopathy: Of "Brain Fog" and "Fusogen Storms".Int J Mol Sci. 2023 Aug 10;24(16):12648. doi: 10.3390/ijms241612648. Int J Mol Sci. 2023. PMID: 37628830 Free PMC article. Review.
-
Impact of Long COVID on Lung Function in Children.Medeni Med J. 2024 Jun 28;39(2):74-84. doi: 10.4274/MMJ.galenos.2024.15853. Medeni Med J. 2024. PMID: 38940402 Free PMC article.
-
Effects of a plank-based strength training programme on muscle activation in patients with long COVID: a case series.An Sist Sanit Navar. 2024 Oct 2;47(3):e1083. doi: 10.23938/ASSN.1083. An Sist Sanit Navar. 2024. PMID: 39364804 Free PMC article.
-
Advance in the mechanism and clinical research of myalgia in long COVID.Am J Clin Exp Immunol. 2024 Aug 25;13(4):142-164. doi: 10.62347/TXVO6284. eCollection 2024. Am J Clin Exp Immunol. 2024. PMID: 39310121 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

