Gluconate Kinase Is Required for Gluconate Assimilation and Sporulation in Cryptococcus neoformans
- PMID: 35412378
- PMCID: PMC9045243
- DOI: 10.1128/spectrum.00301-22
Gluconate Kinase Is Required for Gluconate Assimilation and Sporulation in Cryptococcus neoformans
Abstract
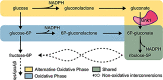
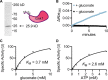
Cryptococcus neoformans is an environmental yeast and an opportunistic human pathogen. The ability to cause disease depends on the ability to adapt to the human host. Previous studies implicated
Keywords: Cryptococcus neoformans; carbon dioxide; gluconate kinase; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Phenotypic characterization of HAM1, a novel mating regulator of the fungal pathogen Cryptococcus neoformans.Microbiol Spectr. 2024 Jul 2;12(7):e0341923. doi: 10.1128/spectrum.03419-23. Epub 2024 Jun 6. Microbiol Spectr. 2024. PMID: 38842336 Free PMC article.
-
Amoeba Predation of Cryptococcus neoformans Results in Pleiotropic Changes to Traits Associated with Virulence.mBio. 2021 Apr 27;12(2):e00567-21. doi: 10.1128/mBio.00567-21. mBio. 2021. PMID: 33906924 Free PMC article.
-
Cdk8 and Ssn801 Regulate Oxidative Stress Resistance and Virulence in Cryptococcus neoformans.mBio. 2019 Feb 12;10(1):e02818-18. doi: 10.1128/mBio.02818-18. mBio. 2019. PMID: 30755515 Free PMC article.
-
The interplay of phenotype and genotype in Cryptococcus neoformans disease.Biosci Rep. 2020 Oct 30;40(10):BSR20190337. doi: 10.1042/BSR20190337. Biosci Rep. 2020. PMID: 33021310 Free PMC article. Review.
-
Deciphering the model pathogenic fungus Cryptococcus neoformans.Nat Rev Microbiol. 2005 Oct;3(10):753-64. doi: 10.1038/nrmicro1245. Nat Rev Microbiol. 2005. PMID: 16132036 Review.
Cited by
-
Survival in macrophages induces enhanced virulence in Cryptococcus.mSphere. 2024 Jan 30;9(1):e0050423. doi: 10.1128/msphere.00504-23. Epub 2023 Dec 11. mSphere. 2024. PMID: 38073033 Free PMC article.
-
Last but not yeast-The many forms of Cryptococcus neoformans.PLoS Pathog. 2023 Jan 5;19(1):e1011048. doi: 10.1371/journal.ppat.1011048. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36602969 Free PMC article. No abstract available.
-
Comprehensive genome-scale metabolic model of the human pathogen Cryptococcus neoformans: A platform for understanding pathogen metabolism and identifying new drug targets.Front Bioinform. 2023 Jan 13;3:1121409. doi: 10.3389/fbinf.2023.1121409. eCollection 2023. Front Bioinform. 2023. PMID: 36714093 Free PMC article.
References
-
- Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, Geddes JMH, Griffiths EJ, Choi J, Cadieux B, Caza M, Attarian R. 2012. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell 11:109–118. doi:10.1128/EC.05273-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

