A cross-sectional examination of conflict-of-interest disclosures of physician-authors publishing in high-impact US medical journals
- PMID: 35410932
- PMCID: PMC9021780
- DOI: 10.1136/bmjopen-2021-057598
A cross-sectional examination of conflict-of-interest disclosures of physician-authors publishing in high-impact US medical journals
Abstract
Objective: To assess the accuracy of self-reported financial conflict-of-interest (COI) disclosures in the New England Journal of Medicine (NEJM) and the Journal of the American Medical Association (JAMA) within the requisite disclosure period prior to article submission.
Design: Cross-sectional investigation.
Data sources: Original clinical-trial research articles published in NEJM (n=206) or JAMA (n=188) from 1 January 2017 to 31 December 2017; self-reported COI disclosure forms submitted to NEJM or JAMA with the authors' published articles; Open Payments website (from database inception; latest search: August 2019).
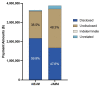
Main outcome measures: Financial data reported to Open Payments from 2014 to 2016 (a time period that included all subjects' requisite disclosure windows) were compared with self-reported disclosure forms submitted to the journals. Payments selected for analysis were defined by Open Payments as 'general payments.' Payment types were categorised as 'disclosed,' 'undisclosed,' 'indeterminate' or 'unrelated'.
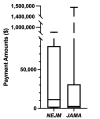
Results: Thirty-one articles from NEJM and 31 articles from JAMA met inclusion criteria. The physician-authors (n=118) received a combined total of US$7.48 million. Of the 106 authors (89.8%) who received payments, 86 (81.1%) received undisclosed payments. The top 23 most highly compensated received US$6.32 million, of which US$3.00 million (47.6%) was undisclosed.
Conclusions: High payment amounts, as well as high proportions of undisclosed financial compensation, regardless of amount received, comprised potential COIs for two influential US medical journals. Further research is needed to explain why such high proportions of general payments were undisclosed and whether journals that rely on self-reported COI disclosure need to reconsider their policies.
Keywords: CLINICAL PHARMACOLOGY; Clinical trials; MEDICAL ETHICS.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BJP is part of an osteoarthritis research team supported by Pfizer and Eli Lilly. The authors have no personal or institutional interest with regards to the authorship and/or publication of this manuscript.
Figures
Similar articles
-
Financial conflicts of interest among US physician authors of 2020 clinical practice guidelines: a cross-sectional study.BMJ Open. 2023 Jan 23;13(1):e069115. doi: 10.1136/bmjopen-2022-069115. BMJ Open. 2023. PMID: 36690402 Free PMC article.
-
Conflict of interest disclosure in orthopaedic and general surgical trauma literature.Injury. 2021 Aug;52(8):2148-2153. doi: 10.1016/j.injury.2021.03.011. Epub 2021 Mar 7. Injury. 2021. PMID: 33812702
-
Accuracy of Financial Disclosures in Radiology Journals.J Am Coll Radiol. 2024 Sep;21(9):1527-1533. doi: 10.1016/j.jacr.2024.01.027. Epub 2024 Mar 23. J Am Coll Radiol. 2024. PMID: 38527639
-
Conflict of Interest Disclosure in a Top-Tier Portuguese Medical Journal.Acta Med Port. 2017 Sep 29;30(9):652-655. doi: 10.20344/amp.8458. Acta Med Port. 2017. PMID: 29025532 Review.
-
Reviewing the Reviewers Potential Financial Conflicts of Interest in Editorial Boards of Surgery Journals.Ann Surg. 2022 Dec 1;276(6):e1089-e1094. doi: 10.1097/SLA.0000000000004929. Epub 2021 Jun 2. Ann Surg. 2022. PMID: 34091509 Review.
Cited by
-
Payments by Drug and Medical Device Manufacturers to US Peer Reviewers of Major Medical Journals.JAMA. 2024 Oct 10;332(17):1480-2. doi: 10.1001/jama.2024.17681. Online ahead of print. JAMA. 2024. PMID: 39388188
-
Discrepancies in Conflict-of-Interest Disclosures Among Physicians Receiving Compensation for Monoclonal Antibody Drugs.J Gen Intern Med. 2024 May;39(7):1135-1141. doi: 10.1007/s11606-023-08523-7. Epub 2023 Nov 14. J Gen Intern Med. 2024. PMID: 37962731
-
How are declarations of interest working? A cross-sectional study in declarations of interest in healthcare practice in Scotland and England in 2020/2021.BMJ Open. 2022 Nov 4;12(11):e065365. doi: 10.1136/bmjopen-2022-065365. BMJ Open. 2022. PMID: 36332951 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
