M2-like macrophages transplantation protects against the doxorubicin-induced heart failure via mitochondrial transfer
- PMID: 35410296
- PMCID: PMC8996664
- DOI: 10.1186/s40824-022-00260-y
M2-like macrophages transplantation protects against the doxorubicin-induced heart failure via mitochondrial transfer
Abstract
Aims: The alternatively activated macrophages have shown a cardioprotective effect in heart failure. However, the effect of M2 adoptive transfer in non-ischemic heart failure is unknown. In this study, we evaluated the efficacy of M-CSF plus IL-4 induced M2-like macrophages transplantation in doxorubicin-induced cardiotoxicity.
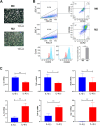
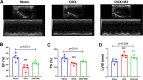
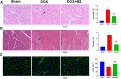
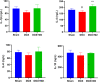
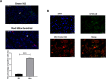
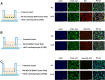
Methods: Bone marrow mononuclear cells were polarized as CCR2+CD206+ M2-like macrophages by a combination of M-CSF plus IL-4 treatment. C57BL/6 mice received a single intraperitoneal injection of doxorubicin (15 mg/kg). The treatment group were treated with M2-like macrophages (1 × 10^6 cells per mouse; i.v.) once a week for 2 weeks. After 3 weeks, we examined the percentage of resident cells and cardiac function. Furthermore, we evaluated cardiac fibrosis, cardiomyocyte apoptosis and circulating inflammatory factors. Finally, we investigated the mitochondria transfer in vitro in a direct and indirect co-culture conditions.
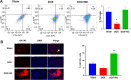
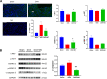
Results: Cardiac function was significantly improved in doxorubicin-induced heart failure by adoptive transfer of M2-like macrophages. Besides, M2-like macrophages treatment attenuated cardiac fibrosis and cardiomyocyte apoptosis, as well as increased the level of circulating IL-4 and Th2 response. In vitro, M2-like macrophages could transfer mitochondria to injured cardiomyocytes in a direct and indirect way.
Conclusions: In our study, adoptive transfer of M2-like macrophages could protect against the doxorubicin-induced cardiotoxicity, which may be partly attributed to mitochondria transfer. And M2-like macrophages transplantation could become a treatment for non-ischemic heart failure in the clinical practice.
Keywords: Cardiotoxicity; Cell transplantation; Heart failure; M2-like macrophages; Mitochondria.
© 2022. The Author(s).
Conflict of interest statement
There is no competing interest to declare.
Figures
Similar articles
-
Multi-walled carbon nanotubes exacerbate doxorubicin-induced cardiotoxicity by altering gut microbiota and pulmonary and colonic macrophage phenotype in mice.Toxicology. 2020 Apr 15;435:152410. doi: 10.1016/j.tox.2020.152410. Epub 2020 Feb 14. Toxicology. 2020. PMID: 32068018
-
Failed renoprotection by alternatively activated bone marrow macrophages is due to a proliferation-dependent phenotype switch in vivo.Kidney Int. 2014 Apr;85(4):794-806. doi: 10.1038/ki.2013.341. Epub 2013 Sep 18. Kidney Int. 2014. PMID: 24048378
-
Cardiomyocyte-specific disruption of Cathepsin K protects against doxorubicin-induced cardiotoxicity.Cell Death Dis. 2018 Jun 7;9(6):692. doi: 10.1038/s41419-018-0727-2. Cell Death Dis. 2018. PMID: 29880809 Free PMC article.
-
Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy.Cells. 2019 Oct 9;8(10):1224. doi: 10.3390/cells8101224. Cells. 2019. PMID: 31600901 Free PMC article.
-
M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T-cell proliferation.Immunology. 2016 Nov;149(3):320-328. doi: 10.1111/imm.12647. Epub 2016 Aug 9. Immunology. 2016. PMID: 27421990 Free PMC article.
Cited by
-
A longitudinal evaluation of oxidative stress - mitochondrial dysfunction - ferroptosis genes in anthracycline-induced cardiotoxicity.BMC Cardiovasc Disord. 2024 Jul 10;24(1):350. doi: 10.1186/s12872-024-03967-z. BMC Cardiovasc Disord. 2024. PMID: 38987722 Free PMC article.
-
Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner.Toxins (Basel). 2023 Apr 19;15(4):298. doi: 10.3390/toxins15040298. Toxins (Basel). 2023. PMID: 37104236 Free PMC article.
-
Immunomodulation for Tissue Repair and Regeneration.Tissue Eng Regen Med. 2023 Jun;20(3):389-409. doi: 10.1007/s13770-023-00525-0. Epub 2023 Mar 15. Tissue Eng Regen Med. 2023. PMID: 36920675 Free PMC article. Review.
-
Macrophages in cardiovascular diseases: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 May 31;9(1):130. doi: 10.1038/s41392-024-01840-1. Signal Transduct Target Ther. 2024. PMID: 38816371 Free PMC article. Review.
-
Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms, Clinical Management and Innovative Treatment.Drug Des Devel Ther. 2024 Sep 12;18:4089-4116. doi: 10.2147/DDDT.S469331. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39286288 Free PMC article. Review.
References
-
- Narayan HK, Finkelman B, French B, et al. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation. 2017;135:1397–412. doi: 10.1161/CIRCULATIONAHA.116.023463. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

