Trimethylamine N-Oxide (TMAO) Impairs Purinergic Induced Intracellular Calcium Increase and Nitric Oxide Release in Endothelial Cells
- PMID: 35409341
- PMCID: PMC8999849
- DOI: 10.3390/ijms23073982
Trimethylamine N-Oxide (TMAO) Impairs Purinergic Induced Intracellular Calcium Increase and Nitric Oxide Release in Endothelial Cells
Abstract
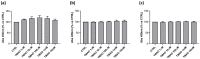
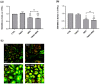
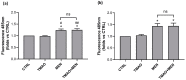
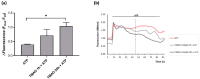
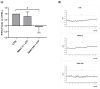
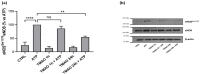
Trimethylamine N-oxide (TMAO) is a diet derived compound directly introduced through foodstuff, or endogenously synthesized from its precursors, primarily choline, L-carnitine, and ergothioneine. New evidence outlines high TMAO plasma concentrations in patients with overt cardiovascular disease, but its direct role in pathological development is still controversial. The purpose of the study was to evaluate the role of TMAO in affecting key intracellular factors involved in endothelial dysfunction development, such as reactive oxygen species, mitochondrial health, calcium balance, and nitric oxide release using bovine aortic endothelial cells (BAE-1). Cell viability and oxidative stress indicators were monitored after acute and prolonged TMAO treatment. The role of TMAO in interfering with the physiological purinergic vasodilatory mechanism after ATP stimulation was defined through measurements of the rise of intracellular calcium, nitric oxide release, and eNOS phosphorylation at Ser1179 (eNOSSer1179). TMAO was not cytotoxic for BAE-1 and it did not induce the rise of reactive oxygen species and impairment of mitochondrial membrane potential, either in the basal condition or in the presence of a stressor. In contrast, TMAO modified the purinergic response affecting intracellular ATP-induced calcium increase, nitric oxide release, and eNOSSer1179. Results obtained suggest a possible implication of TMAO in impairing the endothelial-dependent vasodilatory mechanism.
Keywords: TMAO; calcium; endothelial dysfunction; endothelial nitric oxide synthase; nitric oxide; trimethylamine N-oxide; vasodilatory mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Endothelial Cell Autophagy Maintains Shear Stress-Induced Nitric Oxide Generation via Glycolysis-Dependent Purinergic Signaling to Endothelial Nitric Oxide Synthase.Arterioscler Thromb Vasc Biol. 2017 Sep;37(9):1646-1656. doi: 10.1161/ATVBAHA.117.309510. Epub 2017 Jul 6. Arterioscler Thromb Vasc Biol. 2017. PMID: 28684613 Free PMC article.
-
Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells.Int J Mol Sci. 2024 Mar 16;25(6):3389. doi: 10.3390/ijms25063389. Int J Mol Sci. 2024. PMID: 38542360 Free PMC article.
-
Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome.Biochem Biophys Res Commun. 2016 Dec 2;481(1-2):63-70. doi: 10.1016/j.bbrc.2016.11.017. Epub 2016 Nov 8. Biochem Biophys Res Commun. 2016. PMID: 27833015
-
Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease.Curr Atheroscler Rep. 2021 Feb 17;23(4):12. doi: 10.1007/s11883-021-00910-x. Curr Atheroscler Rep. 2021. PMID: 33594574 Review.
-
Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far?Eur J Nutr. 2021 Oct;60(7):3567-3584. doi: 10.1007/s00394-021-02491-6. Epub 2021 Feb 3. Eur J Nutr. 2021. PMID: 33533968 Review.
Cited by
-
Dietary calcium, phosphorus, and potassium intake associated with erectile dysfunction in the National Health and Nutrition Examination Survey (NHANES) 2001 to 2004.PLoS One. 2024 Feb 21;19(2):e0297129. doi: 10.1371/journal.pone.0297129. eCollection 2024. PLoS One. 2024. PMID: 38381721 Free PMC article.
-
Research progress in the relationship between gut microbiota metabolite trimethylamine N-oxide and ischemic stroke.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Mar 28;49(3):447-456. doi: 10.11817/j.issn.1672-7347.2024.230427. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38970519 Free PMC article. Review. Chinese, English.
-
The gut microbiota in thrombosis.Nat Rev Cardiol. 2025 Feb;22(2):121-137. doi: 10.1038/s41569-024-01070-6. Epub 2024 Sep 17. Nat Rev Cardiol. 2025. PMID: 39289543 Review.
-
Trimethylamine-N-oxide promotes osteoclast differentiation and oxidative stress by activating NF-κB pathway.Aging (Albany NY). 2024 May 27;16(10):9251-9263. doi: 10.18632/aging.205869. Epub 2024 May 27. Aging (Albany NY). 2024. PMID: 38809508 Free PMC article.
-
Gut Failure: A Review of the Pathophysiology and Therapeutic Potentials in the Gut-Heart Axis.J Clin Med. 2023 Mar 29;12(7):2567. doi: 10.3390/jcm12072567. J Clin Med. 2023. PMID: 37048650 Free PMC article. Review.

