The Mammary Gland: Basic Structure and Molecular Signaling during Development
- PMID: 35409243
- PMCID: PMC8998991
- DOI: 10.3390/ijms23073883
The Mammary Gland: Basic Structure and Molecular Signaling during Development
Abstract
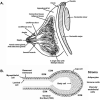
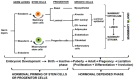
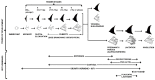
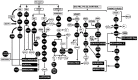
The mammary gland is a compound, branched tubuloalveolar structure and a major characteristic of mammals. The mammary gland has evolved from epidermal apocrine glands, the skin glands as an accessory reproductive organ to support postnatal survival of offspring by producing milk as a source of nutrition. The mammary gland development begins during embryogenesis as a rudimentary structure that grows into an elementary branched ductal tree and is embedded in one end of a larger mammary fat pad at birth. At the onset of ovarian function at puberty, the rudimentary ductal system undergoes dramatic morphogenetic change with ductal elongation and branching. During pregnancy, the alveolar differentiation and tertiary branching are completed, and during lactation, the mature milk-producing glands eventually develop. The early stages of mammary development are hormonal independent, whereas during puberty and pregnancy, mammary gland development is hormonal dependent. We highlight the current understanding of molecular regulators involved during different stages of mammary gland development.
Keywords: development; mammary gland; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Review: Mammary gland development in swine: embryo to early lactation.Animal. 2019 Jul;13(S1):s11-s19. doi: 10.1017/S1751731119000521. Animal. 2019. PMID: 31280748 Review.
-
Mammary gland development.Wiley Interdiscip Rev Dev Biol. 2012 Jul-Aug;1(4):533-57. doi: 10.1002/wdev.35. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 22844349 Free PMC article. Review.
-
Pubertal mammary gland development: insights from mouse models.J Mammary Gland Biol Neoplasia. 2006 Oct;11(3-4):283-97. doi: 10.1007/s10911-006-9024-2. J Mammary Gland Biol Neoplasia. 2006. PMID: 17089203 Review.
-
Hormonal and local control of mammary branching morphogenesis.Differentiation. 2006 Sep;74(7):365-81. doi: 10.1111/j.1432-0436.2006.00105.x. Differentiation. 2006. PMID: 16916375 Free PMC article. Review.
-
Molecular regulators of pubertal mammary gland development.Ann Med. 2011 May;43(3):212-34. doi: 10.3109/07853890.2011.554425. Epub 2011 Mar 20. Ann Med. 2011. PMID: 21417804 Review.
Cited by
-
Accelerating whole-sample polarization-resolved second harmonic generation imaging in mammary gland tissue via generative adversarial networks.Biomed Opt Express. 2024 Aug 15;15(9):5251-5271. doi: 10.1364/BOE.529779. eCollection 2024 Sep 1. Biomed Opt Express. 2024. PMID: 39296390 Free PMC article.
-
Effects of Hormones on Breast Development and Breast Cancer Risk in Transgender Women.Cancers (Basel). 2022 Dec 30;15(1):245. doi: 10.3390/cancers15010245. Cancers (Basel). 2022. PMID: 36612241 Free PMC article. Review.
-
Reducing Inert Materials for Optimal Cell-Cell and Cell-Matrix Interactions within Microphysiological Systems.Biomimetics (Basel). 2024 Apr 25;9(5):262. doi: 10.3390/biomimetics9050262. Biomimetics (Basel). 2024. PMID: 38786472 Free PMC article.
-
Toward Characterizing Lymphatic Vasculature in the Mammary Gland During Normal Development and Tumor-Associated Remodeling.J Mammary Gland Biol Neoplasia. 2024 Jan 13;29(1):1. doi: 10.1007/s10911-023-09554-w. J Mammary Gland Biol Neoplasia. 2024. PMID: 38218743 Free PMC article. Review.
-
Lactation in domestic carnivores.Anim Front. 2023 Jun 14;13(3):71-76. doi: 10.1093/af/vfad027. eCollection 2023 Jun. Anim Front. 2023. PMID: 37324213 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

