Rhein Improves Renal Fibrosis by Restoring Cpt1a-Mediated Fatty Acid Oxidation through SirT1/STAT3/twist1 Pathway
- PMID: 35408745
- PMCID: PMC9000220
- DOI: 10.3390/molecules27072344
Rhein Improves Renal Fibrosis by Restoring Cpt1a-Mediated Fatty Acid Oxidation through SirT1/STAT3/twist1 Pathway
Abstract
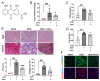
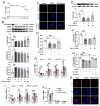
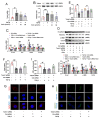
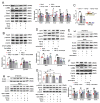
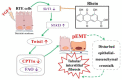
The latest progress in the field of renal fibrosis mainly focuses on the new concept of "partial epithelial-mesenchymal transition (pEMT)" to explain the contribution of renal tubular epithelial (RTE) cells to renal fibrosis and the crucial role of fatty acid oxidation (FAO) dysfunction in RTE cells for the development of renal fibrosis. FAO depression is considered to be secondary or occur simultaneously with pEMT. We explored the relationship between pEMT and FAO and the effect of rhein on them. Intragastric administration of rhein significantly improved the levels of BUN, Scr, α-SMA, collagen 1A and histopathological changes in UUO-rats. Transcriptomic and metabolomic analyses revealed that abnormal signaling pathways were involved in EMT and FAO disorders. RTE cell experiments showed that TGF-β could inhibit the activity of Cpt1a, resulting in ATP depletion and lipid deposition. Cpt1a inhibitor induced EMT, while Cpt1 substrate or rhein inhibited EMT, indicating that Cpt1a-mediated FAO dysfunction is essential for RTE cells EMT. Further studies showed that Cpt1a activity were regulated by SirT1/STAT3/Twist1 pathway. Rhein inhibits RTE cell EMT by promoting Cpt1a-mediated FAO through the SirT1/STAT3/Twist1 pathway. Surprisingly and importantly, our experiments showed that FAO depression occurs before EMT, and EMT is one of the results of FAO depression.
Keywords: Cpt1a-mediated fatty acid oxidation; SirT1/STAT3 pathway; Twist1; epithelial–mesenchymal transition; renal fibrosis; rhein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of the CTRP6/AMPK pathway in kidney fibrosis through the promotion of fatty acid oxidation.Eur J Pharmacol. 2021 Feb 5;892:173755. doi: 10.1016/j.ejphar.2020.173755. Epub 2020 Nov 25. Eur J Pharmacol. 2021. PMID: 33245899
-
Cryptotanshinone alleviates liver fibrosis via inhibiting STAT3/CPT1A-dependent fatty acid oxidation in hepatic stellate cells.Chem Biol Interact. 2024 Aug 25;399:111119. doi: 10.1016/j.cbi.2024.111119. Epub 2024 Jun 25. Chem Biol Interact. 2024. PMID: 38936533
-
Twist1 downregulation of PGC-1α decreases fatty acid oxidation in tubular epithelial cells, leading to kidney fibrosis.Theranostics. 2022 May 1;12(8):3758-3775. doi: 10.7150/thno.71722. eCollection 2022. Theranostics. 2022. PMID: 35664054 Free PMC article.
-
CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential.Endocrinology. 2020 Feb 1;161(2):bqz046. doi: 10.1210/endocr/bqz046. Endocrinology. 2020. PMID: 31900483 Review.
-
Druggability of lipid metabolism modulation against renal fibrosis.Acta Pharmacol Sin. 2022 Mar;43(3):505-519. doi: 10.1038/s41401-021-00660-1. Epub 2021 May 14. Acta Pharmacol Sin. 2022. PMID: 33990764 Free PMC article. Review.
Cited by
-
Metabolic reprogramming and renal fibrosis: what role might Chinese medicine play?Chin Med. 2024 Oct 28;19(1):148. doi: 10.1186/s13020-024-01004-x. Chin Med. 2024. PMID: 39465434 Free PMC article. Review.
-
Lipid homeostasis in diabetic kidney disease.Int J Biol Sci. 2024 Jul 2;20(10):3710-3724. doi: 10.7150/ijbs.95216. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113692 Free PMC article. Review.
-
Renal Fibrosis: SIRT1 Still of Value.Biomedicines. 2024 Aug 23;12(9):1942. doi: 10.3390/biomedicines12091942. Biomedicines. 2024. PMID: 39335456 Free PMC article. Review.
-
IL-37 Ameliorates Renal Fibrosis by Restoring CPT1A-Mediated Fatty Acid Oxidation in Diabetic Kidney Disease.Kidney Dis (Basel). 2023 Jan 31;9(2):104-117. doi: 10.1159/000529460. eCollection 2023 Apr. Kidney Dis (Basel). 2023. PMID: 37065609 Free PMC article.
-
Twist1 as a target for prevention of cutaneous squamous cell carcinoma.Mol Carcinog. 2023 Jan;62(1):62-76. doi: 10.1002/mc.23482. Epub 2022 Nov 13. Mol Carcinog. 2023. PMID: 36373194 Free PMC article.
References
-
- Mise K., Hoshino J., Ueno T., Hazue R., Hasegawa J., Sekine A., Sumida K., Hiramatsu R., Hasegawa E., Yamanouchi M., et al. Prognostic value of tubulointerstitial lesions, urinary N-acetyl-β-d-glucosaminidase, and urinary β2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. Clin. J. Am. Soc. Nephrol. 2016;11:593–601. doi: 10.2215/CJN.04980515. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

