Protective Effect of Djulis (Chenopodium formosanum) Extract against UV- and AGEs-Induced Skin Aging via Alleviating Oxidative Stress and Collagen Degradation
- PMID: 35408731
- PMCID: PMC9000422
- DOI: 10.3390/molecules27072332
Protective Effect of Djulis (Chenopodium formosanum) Extract against UV- and AGEs-Induced Skin Aging via Alleviating Oxidative Stress and Collagen Degradation
Abstract
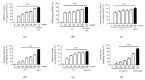
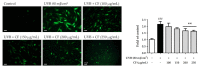
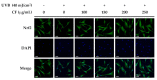
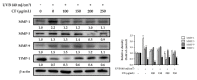
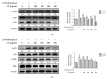
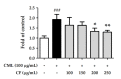
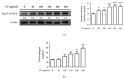
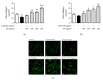
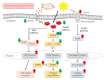
Skin aging is a complex process involving photoaging and glycation stress, which share some fundamental pathways and have common mediators. They can cause skin damage and collagen degradation by inducing oxidative stress and the accumulation of reactive oxygen species (ROS). Chenopodium formosanum (CF), also known as Djulis, is a traditional cereal in Taiwan. This study investigated the protection mechanisms of CF extract against ultraviolet (UV) radiation and advanced glycation end products (AGEs)-induced stress. The results indicated that CF extract had strong antioxidant and free radical scavenging effects. It could reduce UV-induced intracellular ROS generation and initiate the antioxidant defense system by activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway in human skin fibroblasts. CF extract modulated mitogen-activated protein kinase (MAPK) and transformed growth factor-beta (TGF-β) signaling pathways to alleviate oxidative stress-induced skin aging. Moreover, the results revealed that CF extract not only promoted collagen synthesis but also improved aging-induced collagen degradation. CF extract attenuated AGEs-induced ROS production and the upregulation of receptor for AGEs (RAGE). The overall results suggest that CF extract provides an effective anti-aging strategy by preventing skin damage from oxidative stress and collagen loss with potent antioxidant, anti-photoaging, and antiglycation activities.
Keywords: Chenopodium formosanum; Djulis; advanced glycation end products; glycation stress; reactive oxygen species; ultraviolet radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
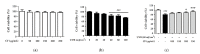



Similar articles
-
Djulis supplementation against oxidative stress and ultraviolet radiation-induced cell damage: The influence of antioxidant status and aging of skin in healthy subjects.J Cosmet Dermatol. 2022 Jul;21(7):2945-2953. doi: 10.1111/jocd.14482. Epub 2021 Oct 12. J Cosmet Dermatol. 2022. PMID: 34636463 Clinical Trial.
-
Djulis (Chenopodium formosanum Koidz.) Water Extract and Its Bioactive Components Ameliorate Dermal Damage in UVB-Irradiated Skin Models.Biomed Res Int. 2016;2016:7368797. doi: 10.1155/2016/7368797. Epub 2016 Oct 26. Biomed Res Int. 2016. PMID: 27847821 Free PMC article.
-
Djulis (Chenopodium formosanum) and Its Bioactive Compounds Protect Human Lung Epithelial A549 Cells from Oxidative Injury Induced by Particulate Matter via Nrf2 Signaling Pathway.Molecules. 2021 Dec 31;27(1):253. doi: 10.3390/molecules27010253. Molecules. 2021. PMID: 35011484 Free PMC article.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
Molecular Mechanisms of Polyphenols in Management of Skin Aging.Curr Aging Sci. 2024;17(3):180-188. doi: 10.2174/0118746098287130240212085507. Curr Aging Sci. 2024. PMID: 39248031 Review.
Cited by
-
Enzymatic Deglycation of Damaged Skin by Means of Combined Treatment of Fructosamine-3-Kinase and Fructosyl-Amino Acid Oxidase.Int J Mol Sci. 2023 May 19;24(10):8981. doi: 10.3390/ijms24108981. Int J Mol Sci. 2023. PMID: 37240327 Free PMC article.
-
Immune mRNA Expression and Fecal Microbiome Composition Change Induced by Djulis (Chenopodium formosanum Koidz.) Supplementation in Aged Mice: A Pilot Study.Medicina (Kaunas). 2024 Sep 20;60(9):1545. doi: 10.3390/medicina60091545. Medicina (Kaunas). 2024. PMID: 39336586 Free PMC article.
-
Regulatory Mechanisms of Natural Active Ingredients and Compounds on Keratinocytes and Fibroblasts in Mitigating Skin Photoaging.Clin Cosmet Investig Dermatol. 2024 Aug 29;17:1943-1962. doi: 10.2147/CCID.S478666. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39224224 Free PMC article. Review.
-
Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway.Int J Mol Sci. 2022 Jun 23;23(13):6960. doi: 10.3390/ijms23136960. Int J Mol Sci. 2022. PMID: 35805987 Free PMC article.
-
Systemic Analyses of Anti-Cell-Senescence Active Compounds in Camellia Sect. Chrysantha Chang and Their Mechanisms.Plants (Basel). 2024 Aug 1;13(15):2139. doi: 10.3390/plants13152139. Plants (Basel). 2024. PMID: 39124256 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

