MMP9: A Tough Target for Targeted Therapy for Cancer
- PMID: 35406619
- PMCID: PMC8998077
- DOI: 10.3390/cancers14071847
MMP9: A Tough Target for Targeted Therapy for Cancer
Abstract
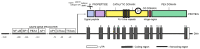
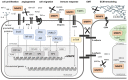
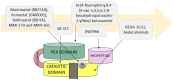
Having the capability to proteolyze diverse structural and signaling proteins, matrix metalloproteinase 9 (MMP9), one of the best-studied secretory endopeptidases, has been identified as a crucial mediator of processes closely associated with tumorigenesis, such as the extracellular matrix reorganization, epithelial to mesenchymal transition, cell migration, new blood vessel formation, and immune response. In this review, we present the current state of knowledge on MMP9 and its role in cancer growth in the context of cell adhesion/migration, cancer-related inflammation, and tumor microenvironment formation. We also summarize recent achievements in the development of selective MMP9 inhibitors and the limitations of using them as anticancer drugs.
Keywords: cancer-related inflammation; epithelial–mesenchymal transmission; extracellular matrix; matrix metalloproteinase 9; targeted therapy.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A Reporter System Evaluates Tumorigenesis, Metastasis, β-catenin/MMP Regulation, and Druggability.Tissue Eng Part A. 2019 Oct;25(19-20):1413-1425. doi: 10.1089/ten.TEA.2018.0348. Epub 2019 Jun 14. Tissue Eng Part A. 2019. PMID: 30734664
-
Matrix metalloproteinase MMP9 maintains epithelial barrier function and preserves mucosal lining in colitis associated cancer.Oncotarget. 2017 Oct 17;8(55):94650-94665. doi: 10.18632/oncotarget.21841. eCollection 2017 Nov 7. Oncotarget. 2017. PMID: 29212256 Free PMC article.
-
Matrix Metalloproteinases (MMPs) in Targeted Drug Delivery: Synthesis of a Potent and Highly Selective Inhibitor against Matrix Metalloproteinase- 7.Curr Top Med Chem. 2020;20(27):2459-2471. doi: 10.2174/1568026620666200722104928. Curr Top Med Chem. 2020. PMID: 32703131 Review.
-
Matrix metalloproteinase 9/gelatinase B is required for neural crest cell migration.Dev Biol. 2012 Apr 15;364(2):162-77. doi: 10.1016/j.ydbio.2012.01.028. Epub 2012 Feb 8. Dev Biol. 2012. PMID: 22342386
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
Cited by
-
MMP9 drives ferroptosis by regulating GPX4 and iron signaling.iScience. 2024 Jul 30;27(9):110622. doi: 10.1016/j.isci.2024.110622. eCollection 2024 Sep 20. iScience. 2024. PMID: 39252956 Free PMC article.
-
Schnurri-3 drives tumor growth and invasion in cancer cells expressing interleukin-13 receptor alpha 2.Cell Death Dis. 2023 Nov 14;14(11):742. doi: 10.1038/s41419-023-06255-4. Cell Death Dis. 2023. PMID: 37963919 Free PMC article.
-
CD44 Is Associated with Poor Prognosis of ccRCC and Facilitates ccRCC Cell Migration and Invasion through HAS1/MMP9.Biomedicines. 2023 Jul 24;11(7):2077. doi: 10.3390/biomedicines11072077. Biomedicines. 2023. PMID: 37509716 Free PMC article.
-
Integrating single-cell and bulk RNA sequencing reveals CK19 + cancer stem cells and their specific SPP1 + tumor-associated macrophage niche in HBV-related hepatocellular carcinoma.Hepatol Int. 2024 Feb;18(1):73-90. doi: 10.1007/s12072-023-10615-9. Epub 2023 Dec 30. Hepatol Int. 2024. PMID: 38159218
-
Stage-Dependent Levels of Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase 9 in the Prognosis of Colorectal Cancer.Biomedicines. 2023 Jun 26;11(7):1839. doi: 10.3390/biomedicines11071839. Biomedicines. 2023. PMID: 37509480 Free PMC article.
References
-
- Augoff K., Hryniewicz-Jankowska A., Tabola R., Czapla L., Szelachowski P., Wierzbicki J., Grabowski K., Sikorski A.F. Upregulated expression and activation of membrane? Associated proteases in esophageal squamous cell carcinoma. Oncol. Rep. 2014;31:2820–2826. doi: 10.3892/or.2014.3162. - DOI - PubMed
-
- Martins L.M., Dourado C.S.D.M.E., Campos-Verdes L.M., Sampaio F.A., Revoredo C.M.S., Costa-Silva D.R., Barros-Oliveira M.D.C., Junior E.D.J.N., Rego-Medeiros L.M.D., Gebrim L.H., et al. Expression of matrix metalloproteinase 2 and 9 in breast cancer and breast fibroadenoma: A randomized, double-blind study. Oncotarget. 2019;10:6879–6884. doi: 10.18632/oncotarget.27347. - DOI - PMC - PubMed
-
- Peltonen R., Hagström J., Tervahartiala T., Sorsa T., Haglund C., Isoniemi H. High Expression of MMP-9 in Primary Tumors and High Preoperative MPO in Serum Predict Improved Prognosis in Colorectal Cancer with Operable Liver Metastases. Oncology. 2021;99:144–160. doi: 10.1159/000510609. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

