E-Cadherin-Deficient Cells Are Sensitive to the Multikinase Inhibitor Dasatinib
- PMID: 35406381
- PMCID: PMC8996982
- DOI: 10.3390/cancers14071609
E-Cadherin-Deficient Cells Are Sensitive to the Multikinase Inhibitor Dasatinib
Abstract
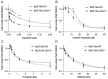
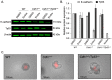
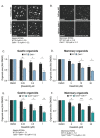
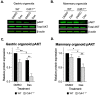
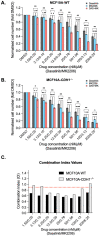
The CDH1 gene, encoding the cell adhesion protein E-cadherin, is one of the most frequently mutated genes in gastric cancer and inactivating germline CDH1 mutations are responsible for the cancer syndrome hereditary diffuse gastric cancer (HDGC). CDH1-deficient gastric cancers exhibit high AKT serine/threonine kinase 3 (AKT3) expression, but specific drugs against this AKT isoform are not available. We therefore used two publicly available datasets to identify AKT3-associated genes which could be used to indirectly target AKT3. Reactome analysis identified an enrichment of extracellular matrix remodelling genes in AKT3-high gastric cancers. Of the 51 genes that were significantly correlated with AKT3 (but not AKT1), discoidin domain receptor tyrosine kinase 2 (DDR2) showed the strongest positive association. Treatment of isogenic human cells and mouse gastric and mammary organoids with dasatinib, a small molecule inhibitor of multiple kinases including SRC, BCR-ABL and DDR2, preferentially slowed the growth and induced apoptosis of E-cadherin-deficient cells. Dasatinib treatment also preferentially slowed the growth of gastric and mammary organoids harbouring both Cdh1 and Tp53 mutations. In organoid models, dasatinib treatment was associated with decreased phosphorylation of total AKT, with a stronger effect seen in Cdh1-deficient organoids. Treatment with combinations of dasatinib and an inhibitor of AKT, MK2206, enhanced the effect of dasatinib in breast MCF10A cells. In conclusion, targeting the DDR2-SRC-AKT3 axis with dasatinib represents a promising approach for the chemoprevention and chemotherapy of gastric and breast cancers lacking E-cadherin.
Keywords: AKT serine/threonine kinase 3 AKT3; E-cadherin; HDGC; chemoprevention; dasatinib; diffuse gastric cancer; discoidin domain receptor 2 (DDR2); lobular breast cancer; synthetic lethality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Allosteric AKT Inhibitors Target Synthetic Lethal Vulnerabilities in E-Cadherin-Deficient Cells.Cancers (Basel). 2019 Sep 13;11(9):1359. doi: 10.3390/cancers11091359. Cancers (Basel). 2019. PMID: 31540244 Free PMC article.
-
E-Cadherin-Deficient Epithelial Cells Are Sensitive to HDAC Inhibitors.Cancers (Basel). 2021 Dec 30;14(1):175. doi: 10.3390/cancers14010175. Cancers (Basel). 2021. PMID: 35008338 Free PMC article.
-
Merging perspectives: genotype-directed molecular therapy for hereditary diffuse gastric cancer (HDGC) and E-cadherin-EGFR crosstalk.Clin Transl Med. 2018 Feb 22;7(1):7. doi: 10.1186/s40169-018-0184-7. Clin Transl Med. 2018. PMID: 29468433 Free PMC article.
-
CDH1 and hereditary diffuse gastric cancer: a narrative review.Chin Clin Oncol. 2023 Jun;12(3):25. doi: 10.21037/cco-23-36. Epub 2023 Jun 5. Chin Clin Oncol. 2023. PMID: 37303221 Review.
-
CDH1 (E-Cadherin) Mutation and Gastric Cancer: Genetics, Molecular Mechanisms and Guidelines for Management.Cancer Manag Res. 2019 Dec 13;11:10477-10486. doi: 10.2147/CMAR.S208818. eCollection 2019. Cancer Manag Res. 2019. PMID: 31853199 Free PMC article. Review.
Cited by
-
Non-targeted biopsies in hereditary diffuse gastric cancer: Necessary, but not enough.Transl Cancer Res. 2023 Jul 31;12(7):1883-1886. doi: 10.21037/tcr-23-541. Epub 2023 Jul 6. Transl Cancer Res. 2023. PMID: 37588744 Free PMC article. No abstract available.
-
Hereditary colorectal, gastric, and pancreatic cancer: comprehensive review.BJS Open. 2023 May 5;7(3):zrad023. doi: 10.1093/bjsopen/zrad023. BJS Open. 2023. PMID: 37165697 Free PMC article. Review.
-
Current advances in understanding the molecular profile of hereditary diffuse gastric cancer and its clinical implications.J Exp Clin Cancer Res. 2023 Mar 4;42(1):57. doi: 10.1186/s13046-023-02622-3. J Exp Clin Cancer Res. 2023. PMID: 36869400 Free PMC article. Review.
-
Toxoplasma gondii suppresses proliferation and migration of breast cancer cells by regulating their transcriptome.Cancer Cell Int. 2024 Apr 23;24(1):144. doi: 10.1186/s12935-024-03333-1. Cancer Cell Int. 2024. PMID: 38654350 Free PMC article.
References
-
- Blair V.R., McLeod M., Carneiro F., Coit D.G., D’Addario J.L., van Dieren J.M., Harris K.L., Hoogerbrugge N., Oliveira C., van der Post R.S., et al. Hereditary Diffuse Gastric Cancer: Updated Clinical Practice Guidelines. Lancet Oncol. 2020;21:e386–e397. doi: 10.1016/S1470-2045(20)30219-9. - DOI - PMC - PubMed
-
- Roberts M.E., Ranola J.M.O., Marshall M.L., Susswein L.R., Graceffo S., Bohnert K., Tsai G., Klein R.T., Hruska K.S., Shirts B.H. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol. 2019;5:1325–1331. doi: 10.1001/jamaoncol.2019.1208. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

