Efficiency of bis-amidate phosphonate prodrugs
- PMID: 35405283
- PMCID: PMC10245299
- DOI: 10.1016/j.bmcl.2022.128724
Efficiency of bis-amidate phosphonate prodrugs
Abstract
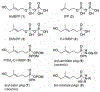
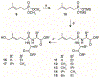
Bis-amidate derivatives have been viewed as attractive phosphonate prodrug forms because of their straightforward synthesis, lack of phosphorus stereochemistry, plasma stability and nontoxic amino acid metabolites. However, the efficiency of bis-amidate prodrug forms is unclear, as prior studies on this class of prodrugs have not evaluated their activation kinetics. Here, we synthetized a small panel of bis-amidate prodrugs of butyrophilin ligands as potential immunotherapy agents. These compounds were examined relative to other prodrug forms delivering the same payload for their stability in plasma and cell lysate, their ability to stimulate T cell proliferation in human PBMCs, and their activation kinetics in a leukemia co-culture model of T cell cytokine production. The bis-amidate prodrugs demonstrate high plasma stability and improved cellular phosphoantigen activity relative to the free phosphonic acid. However, the efficiency of bis-amidate activation is low relative to other prodrugs that contain at least one ester such as aryl-amidate, aryl-acyloxyalkyl ester, and bis-acyloxyalkyl ester forms. Therefore, bis-amidate prodrugs do not drive rapid cellular payload accumulation and they would be more useful for payloads in which slower, sustained-release kinetics are preferred.
Keywords: Butyrophilin; Isoprenoid; Ligand; Phosphoantigen; Phosphonamidate prodrug.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures
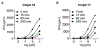
Similar articles
-
Diester Prodrugs of a Phosphonate Butyrophilin Ligand Display Improved Cell Potency, Plasma Stability, and Payload Internalization.J Med Chem. 2023 Nov 23;66(22):15309-15325. doi: 10.1021/acs.jmedchem.3c01358. Epub 2023 Nov 7. J Med Chem. 2023. PMID: 37934915 Free PMC article.
-
Stability and Efficiency of Mixed Aryl Phosphonate Prodrugs.ChemMedChem. 2019 Sep 4;14(17):1597-1603. doi: 10.1002/cmdc.201900344. Epub 2019 Jul 24. ChemMedChem. 2019. PMID: 31226236 Free PMC article.
-
Mixed Aryl Phosphonate Prodrugs of a Butyrophilin Ligand.ACS Med Chem Lett. 2017 Aug 9;8(9):914-918. doi: 10.1021/acsmedchemlett.7b00245. eCollection 2017 Sep 14. ACS Med Chem Lett. 2017. PMID: 28947936 Free PMC article.
-
Novel approaches for designing 5'-O-ester prodrugs of 3'-azido-2', 3'-dideoxythymidine (AZT).Curr Med Chem. 2000 Oct;7(10):995-1039. doi: 10.2174/0929867003374372. Curr Med Chem. 2000. PMID: 10911016 Review.
-
Anti-HIV Nucleoside Phosphonate GS-9148 and Its Prodrug GS-9131: Scale Up of a 2'-F Modified Cyclic Nucleoside Phosphonate and Synthesis of Selected Amidate Prodrugs.Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:14.10.1-21. doi: 10.1002/0471142700.nc1410s56. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25606977 Review.
Cited by
-
Enhanced Plasma Stability and Potency of Aryl/Acyloxy Prodrugs of a BTN3A1 Ligand.ACS Med Chem Lett. 2024 Sep 25;15(10):1771-1777. doi: 10.1021/acsmedchemlett.4c00371. eCollection 2024 Oct 10. ACS Med Chem Lett. 2024. PMID: 39411535 Free PMC article.
-
Diester Prodrugs of a Phosphonate Butyrophilin Ligand Display Improved Cell Potency, Plasma Stability, and Payload Internalization.J Med Chem. 2023 Nov 23;66(22):15309-15325. doi: 10.1021/acs.jmedchem.3c01358. Epub 2023 Nov 7. J Med Chem. 2023. PMID: 37934915 Free PMC article.
References
-
- Ebetino FH; Hogan AM; Sun S; Tsoumpra MK; Duan X; Triffitt JT; Kwaasi AA; Dunford JE; Barnett BL; Oppermann U; Lundy MW; Boyde A; Kashemirov BA; McKenna CE; Russell RG, The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49 (1), 20–33. - PubMed
-
- Jansa P; Baszczynski O; Dracinsky M; Votruba I; Zidek Z; Bahador G; Stepan G; Cihlar T; Mackman R; Holy A; Janeba Z, A novel and efficient one-pot synthesis of symmetrical diamide (bis-amidate) prodrugs of acyclic nucleoside phosphonates and evaluation of their biological activities. Eur. J. Med. Chem 2011, 46 (9), 3748–54. - PubMed
-
- Cesnek M; Jansa P; Smidkova M; Mertlikova-Kaiserova H; Dracinsky M; Brust TF; Pavek P; Trejtnar F; Watts VJ; Janeba Z, Bisamidate Prodrugs of 2-Substituted 9-[2-(Phosphonomethoxy)ethyl]adenine (PMEA, adefovir) as Selective Inhibitors of Adenylate Cyclase Toxin from Bordetella pertussis. ChemMedChem 2015, 10 (8), 1351–64. - PubMed
-
- Houot R; Schultz LM; Marabelle A; Kohrt H, T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer immunology research 2015, 3 (10), 1115–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

