Neurotechnological Approaches to the Diagnosis and Treatment of Alzheimer's Disease
- PMID: 35401082
- PMCID: PMC8989850
- DOI: 10.3389/fnins.2022.854992
Neurotechnological Approaches to the Diagnosis and Treatment of Alzheimer's Disease
Abstract
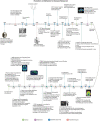
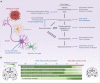
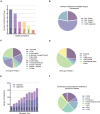
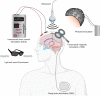
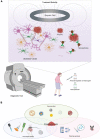
Alzheimer's disease (AD) is the most common cause of dementia in the elderly, clinically defined by progressive cognitive decline and pathologically, by brain atrophy, neuroinflammation, and accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles. Neurotechnological approaches, including optogenetics and deep brain stimulation, have exploded as new tools for not only the study of the brain but also for application in the treatment of neurological diseases. Here, we review the current state of AD therapeutics and recent advancements in both invasive and non-invasive neurotechnologies that can be used to ameliorate AD pathology, including neurostimulation via optogenetics, photobiomodulation, electrical stimulation, ultrasound stimulation, and magnetic neurostimulation, as well as nanotechnologies employing nanovectors, magnetic nanoparticles, and quantum dots. We also discuss the current challenges in developing these neurotechnological tools and the prospects for implementing them in the treatment of AD and other neurodegenerative diseases.
Keywords: Alzheimer’s disease; amyloid; diagnosis; neurotechnologies; therapeutic.
Copyright © 2022 Ning, Jorfi, Patel, Kim and Tanzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Recent update on the heterogeneity of the Alzheimer's disease spectrum.J Neural Transm (Vienna). 2022 Jan;129(1):1-24. doi: 10.1007/s00702-021-02449-2. Epub 2021 Dec 17. J Neural Transm (Vienna). 2022. PMID: 34919190 Review.
-
What is 'Alzheimer's disease'? The neuropathological heterogeneity of clinically defined Alzheimer's dementia.Curr Opin Neurol. 2021 Apr 1;34(2):237-245. doi: 10.1097/WCO.0000000000000912. Curr Opin Neurol. 2021. PMID: 33591030 Review.
-
Treating Alzheimer's disease with brain stimulation: From preclinical models to non-invasive stimulation in humans.Neurosci Biobehav Rev. 2024 Oct;165:105831. doi: 10.1016/j.neubiorev.2024.105831. Epub 2024 Jul 27. Neurosci Biobehav Rev. 2024. PMID: 39074672 Review.
-
Molecular mechanisms in Alzheimer's disease and the impact of physical exercise with advancements in therapeutic approaches.AIMS Neurosci. 2021 Mar 19;8(3):357-389. doi: 10.3934/Neuroscience.2021020. eCollection 2021. AIMS Neurosci. 2021. PMID: 34183987 Free PMC article. Review.
Cited by
-
Spatial orientation, postural control and the vestibular system in healthy elderly and Alzheimer's dementia.PeerJ. 2023 May 2;11:e15040. doi: 10.7717/peerj.15040. eCollection 2023. PeerJ. 2023. PMID: 37151287 Free PMC article. Review.
-
Efficacy of acoustic stimulation techniques on cognitive functions in individuals with Alzheimer's disease-a scoping review.Alzheimers Res Ther. 2024 Aug 1;16(1):174. doi: 10.1186/s13195-024-01544-2. Alzheimers Res Ther. 2024. PMID: 39085956 Free PMC article. Review.
-
Detection of β-amyloid aggregates/plaques in 5xFAD mice by labelled native PLGA nanoparticles: implication in the diagnosis of alzheimer's disease.J Nanobiotechnology. 2023 Jul 10;21(1):216. doi: 10.1186/s12951-023-01957-5. J Nanobiotechnology. 2023. PMID: 37424018 Free PMC article.
-
Transcranial magneto-acoustic stimulation improves spatial memory and modulates hippocampal neural oscillations in a mouse model of Alzheimer's disease.Front Neurosci. 2024 Feb 7;18:1313639. doi: 10.3389/fnins.2024.1313639. eCollection 2024. Front Neurosci. 2024. PMID: 38384480 Free PMC article.
-
Exploring the molecular mechanisms underlying neuroprotective effect of ellagic acid in okadaic acid-induced Alzheimer's phenotype.Metab Brain Dis. 2024 Oct;39(7):1417-1432. doi: 10.1007/s11011-024-01405-9. Epub 2024 Aug 12. Metab Brain Dis. 2024. PMID: 39133454
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

