A Novel Legumain-Like Protease in Macrobrachium nipponense: Identification, Characterization, and Function Analysis in Ovary Maturation
- PMID: 35399931
- PMCID: PMC8987206
- DOI: 10.3389/fendo.2022.858726
A Novel Legumain-Like Protease in Macrobrachium nipponense: Identification, Characterization, and Function Analysis in Ovary Maturation
Abstract
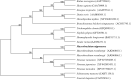
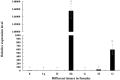
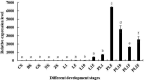
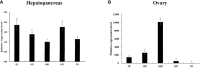
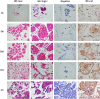
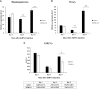
Legumain, also called aspartic endopeptidase (AEP), is a member of the cysteine protease family and is involved in various physiological processes. In this study, we analyzed the characteristics of a novel legumain-like (named Mn-Lel) in the female oriental river prawn, Macrobrachium nipponense, which is involved in ovary maturation. The Mn-Lel is 1,454 bp in length, including a 1,290-bp open reading frame that encodes 430 amino acids. qPCR analysis indicated that Mn-Lel is specifically highly expressed in the hepatopancreas and ovaries of female prawns. It is rarely expressed in embryogenesis, weakly expressed in early larval development stages, and then significantly increased after metamorphosis, which indicated that Mn-Lel is not a maternal gene and mainly plays a role in adults. During the different ovarian stages, Mn-Lel expression in the hepatopancreas had no obvious rules, while its expression in the ovaries had a significant peak in stage III. In situ hybridization studies revealed that Mn-Lel is localized in the oocyte of the ovary. Changes in the gonadosomatic index confirmed the inhibitory effects of Mn-Lel dsRNA on ovary maturation. These results suggest that Mn-Lel has a key role in promoting ovary maturation.
Keywords: Macrobrachium nipponense; RNA interference; legumain-like gene; mRNA expression; ovary maturation.
Copyright © 2022 Jiang, Xiong, Zhang, Zhu, Cheng, Gong, Wu, Qiao and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Molecular Characterization of a Novel Cathepsin L in Macrobrachium nipponense and Its Function in Ovary Maturation.Front Endocrinol (Lausanne). 2022 Jan 10;12:816813. doi: 10.3389/fendo.2021.816813. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35082760 Free PMC article.
-
Cathepsin D Plays a Vital Role in Macrobrachium nipponense of Ovary Maturation: Identification, Characterization, and Function Analysis.Genes (Basel). 2022 Aug 21;13(8):1495. doi: 10.3390/genes13081495. Genes (Basel). 2022. PMID: 36011406 Free PMC article.
-
Molecular characterization and developmental expression of vitellogenin in the oriental river prawn Macrobrachium nipponense and the effects of RNA interference and eyestalk ablation on ovarian maturation.Gene. 2015 May 10;562(1):22-31. doi: 10.1016/j.gene.2014.12.008. Epub 2014 Dec 8. Gene. 2015. PMID: 25499697
-
Function analysis and molecular characterization of cyclin A in ovary development of oriental river prawn, Macrobrachium nipponense.Gene. 2021 Jul 1;788:145583. doi: 10.1016/j.gene.2021.145583. Epub 2021 Mar 20. Gene. 2021. PMID: 33753150
-
Potential Functions of Gem-Associated Protein 2-Like Isoform X1 in the Oriental River Prawn Macrobrachium nipponense: Cloning, qPCR, In Situ Hybridization, and RNAi Analysis.Int J Mol Sci. 2019 Aug 16;20(16):3995. doi: 10.3390/ijms20163995. Int J Mol Sci. 2019. PMID: 31426338 Free PMC article.
Cited by
-
Hepatopancreas Proteomic Analysis Reveals Key Proteins and Pathways in Regulatory of Ovary Maturation of Macrobrachium nipponense.Animals (Basel). 2023 Mar 8;13(6):977. doi: 10.3390/ani13060977. Animals (Basel). 2023. PMID: 36978518 Free PMC article.
-
Studies on the Relationships between Growth and Gonad Development during First Sexual Maturation of Macrobrachium nipponense and Associated SNPs Screening.Int J Mol Sci. 2024 Jun 27;25(13):7071. doi: 10.3390/ijms25137071. Int J Mol Sci. 2024. PMID: 39000192 Free PMC article.
-
Function Analysis of Cholesterol 7-Desaturase in Ovarian Maturation and Molting in Macrobrachium nipponense: Providing Evidence for Reproductive Molting Progress.Int J Mol Sci. 2023 Apr 8;24(8):6940. doi: 10.3390/ijms24086940. Int J Mol Sci. 2023. PMID: 37108104 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

