Combination microRNA-based cellular reprogramming with paclitaxel enhances therapeutic efficacy in a relapsed and multidrug-resistant model of epithelial ovarian cancer
- PMID: 35399604
- PMCID: PMC8971728
- DOI: 10.1016/j.omto.2022.03.005
Combination microRNA-based cellular reprogramming with paclitaxel enhances therapeutic efficacy in a relapsed and multidrug-resistant model of epithelial ovarian cancer
Abstract
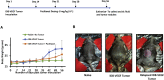
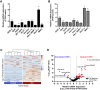
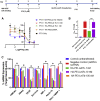
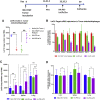
Most advanced-stage ovarian cancer patients, including those with epithelial ovarian cancer (EOC), develop recurrent disease and acquisition of resistance to chemotherapy, leading to limited treatment options. Decrease in Let7b miRNA levels in clinical ovarian cancer has been associated with chemoresistance, increased proliferation, invasion, and relapse in EOC. We have established a murine EOC relapsed model by administering paclitaxel (PTX) and stopping therapy to allow for tumor regrowth. Global microRNA profiling in the relapsed tumor showed significant downregulation of Let7b relative to untreated tumors. Here, we report the use of hyaluronic acid (HA)-based nanoparticle formulation that can deliver Let7b miRNA mimic to tumor cells and achieve cellular programming both in vitro and in vivo. We demonstrate that a therapeutic combination of Let7b miRNA and PTX leads to significant improvement in anti-tumor efficacy in the relapsed model of EOC. We further demonstrate that the combination therapy is safe for repeated administration. This novel approach of cellular reprogramming of tumor cells using clinically relevant miRNA mimic in combination with chemotherapy could enable more effective therapeutic outcomes for patients with advanced-stage relapsed EOC.
Keywords: IDG-VEGF; hyaluronic-acid-based nanoparticles; microRNA-Let7b; ovarian cancer; relapsed/MDR model.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Figures


Similar articles
-
CD44-Targeting PLGA Nanoparticles Incorporating Paclitaxel and FAK siRNA Overcome Chemoresistance in Epithelial Ovarian Cancer.Cancer Res. 2018 Nov 1;78(21):6247-6256. doi: 10.1158/0008-5472.CAN-17-3871. Epub 2018 Aug 16. Cancer Res. 2018. PMID: 30115698
-
Ovarian cancer targeted hyaluronic acid-based nanoparticle system for paclitaxel delivery to overcome drug resistance.Drug Deliv. 2016 Jun;23(5):1810-7. doi: 10.3109/10717544.2015.1101792. Epub 2015 Nov 4. Drug Deliv. 2016. PMID: 26530693
-
Role of hsa‑miR‑105 during the pathogenesis of paclitaxel resistance and its clinical implication in ovarian cancer.Oncol Rep. 2021 May;45(5):84. doi: 10.3892/or.2021.8035. Epub 2021 Apr 13. Oncol Rep. 2021. PMID: 33846814 Free PMC article.
-
Efficacy of trebananib (AMG 386) in treating epithelial ovarian cancer.Expert Opin Pharmacother. 2016;17(6):853-60. doi: 10.1517/14656566.2016.1161027. Epub 2016 Mar 21. Expert Opin Pharmacother. 2016. PMID: 26933765 Review.
-
Luteinising hormone releasing hormone (LHRH) agonists for the treatment of relapsed epithelial ovarian cancer.Cochrane Database Syst Rev. 2016 Jun 29;2016(6):CD011322. doi: 10.1002/14651858.CD011322.pub2. Cochrane Database Syst Rev. 2016. PMID: 27356090 Free PMC article. Review.
Cited by
-
MiR-223-3p in Cancer Development and Cancer Drug Resistance: Same Coin, Different Faces.Int J Mol Sci. 2024 Jul 26;25(15):8191. doi: 10.3390/ijms25158191. Int J Mol Sci. 2024. PMID: 39125761 Free PMC article. Review.
-
Transcriptome profiling and characterization of peritoneal metastasis ovarian cancer xenografts in humanized mice.Sci Rep. 2024 May 24;14(1):11894. doi: 10.1038/s41598-024-60501-z. Sci Rep. 2024. PMID: 38789484 Free PMC article.
-
Current strategies for early epithelial ovarian cancer detection using miRNA as a potential tool.Front Mol Biosci. 2024 Apr 16;11:1361601. doi: 10.3389/fmolb.2024.1361601. eCollection 2024. Front Mol Biosci. 2024. PMID: 38690293 Free PMC article. Review.
-
Development and Perspectives: Multifunctional Nucleic Acid Nanomedicines for Treatment of Gynecological Cancers.Small. 2024 Oct;20(41):e2301776. doi: 10.1002/smll.202301776. Epub 2023 Jul 30. Small. 2024. PMID: 37518857 Review.
-
Engineering of inhalable nano-in-microparticles for co-delivery of small molecules and miRNAs.Discov Nano. 2023 Mar 10;18(1):38. doi: 10.1186/s11671-023-03781-0. Discov Nano. 2023. PMID: 37382704 Free PMC article.
References
-
- Siegel R.L., Miller K.D., Jemal A., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2021;67:7–30. - PubMed
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Bishop K., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., Mariotto A., et al. National Cancer Institute; 2017. SEER Cancer Statistics Review, 1975-2014.
-
- Patch A.M., Christie E.L., Etemadmoghadam D., Garsed D.W., George J., Fereday S., Nones K., Cowin P., Alsop K., Bailey P.J., et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. - PubMed
LinkOut - more resources
Full Text Sources

