p38 MAPK priming boosts VSMC proliferation and arteriogenesis by promoting PGC1α-dependent mitochondrial dynamics
- PMID: 35396524
- PMCID: PMC8994030
- DOI: 10.1038/s41598-022-09757-x
p38 MAPK priming boosts VSMC proliferation and arteriogenesis by promoting PGC1α-dependent mitochondrial dynamics
Abstract
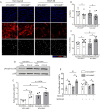
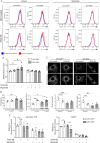
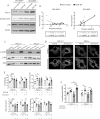
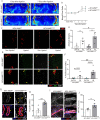
Vascular smooth muscle cell (VSMC) proliferation is essential for arteriogenesis to restore blood flow after artery occlusion, but the mechanisms underlying this response remain unclear. Based on our previous findings showing increased VSMC proliferation in the neonatal aorta of mice lacking the protease MT4-MMP, we aimed at discovering new players in this process. We demonstrate that MT4-MMP absence boosted VSMC proliferation in vitro in response to PDGF-BB in a cell-autonomous manner through enhanced p38 MAPK activity. Increased phospho-p38 in basal MT4-MMP-null VSMCs augmented the rate of mitochondrial degradation by promoting mitochondrial morphological changes through the co-activator PGC1α as demonstrated in PGC1α-/- VSMCs. We tested the in vivo implications of this pathway in a novel conditional mouse line for selective MT4-MMP deletion in VSMCs and in mice pre-treated with the p38 MAPK activator anisomycin. Priming of p38 MAPK activity in vivo by the absence of the protease MT4-MMP or by anisomycin treatment led to enhanced arteriogenesis and improved flow recovery after femoral artery occlusion. These findings may open new therapeutic opportunities for peripheral vascular diseases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Molecular Mechanisms Driven by MT4-MMP in Cancer Progression.Int J Mol Sci. 2023 Jun 9;24(12):9944. doi: 10.3390/ijms24129944. Int J Mol Sci. 2023. PMID: 37373092 Free PMC article. Review.
-
MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway.Exp Cell Res. 2019 Jun 15;379(2):214-224. doi: 10.1016/j.yexcr.2019.03.033. Epub 2019 Mar 29. Exp Cell Res. 2019. PMID: 30930138
-
Angiotensin III induces p38 Mitogen-activated protein kinase leading to proliferation of vascular smooth muscle cells.Pharmacol Rep. 2020 Feb;72(1):246-253. doi: 10.1007/s43440-019-00035-8. Epub 2020 Jan 8. Pharmacol Rep. 2020. PMID: 32016850
-
The inhibitory effect of dexamethasone on platelet-derived growth factor-induced vascular smooth muscle cell migration through up-regulating PGC-1α expression.Exp Cell Res. 2011 May 1;317(8):1083-92. doi: 10.1016/j.yexcr.2010.10.006. Epub 2010 Oct 15. Exp Cell Res. 2011. PMID: 20955697
-
Role of Vascular Smooth Muscle Cell Phenotype Switching in Arteriogenesis.Int J Mol Sci. 2021 Sep 30;22(19):10585. doi: 10.3390/ijms221910585. Int J Mol Sci. 2021. PMID: 34638923 Free PMC article. Review.
Cited by
-
Identification of the microRNA alterations in extracellular vesicles derived from human haemorrhoids.Exp Physiol. 2023 May;108(5):752-761. doi: 10.1113/EP090549. Epub 2023 Jan 9. Exp Physiol. 2023. PMID: 36621805 Free PMC article.
-
Molecular Mechanisms Driven by MT4-MMP in Cancer Progression.Int J Mol Sci. 2023 Jun 9;24(12):9944. doi: 10.3390/ijms24129944. Int J Mol Sci. 2023. PMID: 37373092 Free PMC article. Review.
-
Comparative efficacy of the five most common traditional Chinese medicine monomers in reducing intimal hyperproliferation in arterial balloon injury models: A network meta-analysis.Heliyon. 2024 Aug 19;10(17):e36327. doi: 10.1016/j.heliyon.2024.e36327. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39263082 Free PMC article.
-
Exploring the potential mechanism of Simiao Yongan decoction in the treatment of diabetic peripheral vascular disease based on network pharmacology and molecular docking technology.Medicine (Baltimore). 2023 Dec 29;102(52):e36762. doi: 10.1097/MD.0000000000036762. Medicine (Baltimore). 2023. PMID: 38206683 Free PMC article.
-
Mitochondrial dynamics in vascular remodeling and target-organ damage.Front Cardiovasc Med. 2023 Feb 13;10:1067732. doi: 10.3389/fcvm.2023.1067732. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36860274 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

