Membrane-actin interactions in morphogenesis: Lessons learned from Drosophila cellularization
- PMID: 35396167
- PMCID: PMC9532467
- DOI: 10.1016/j.semcdb.2022.03.028
Membrane-actin interactions in morphogenesis: Lessons learned from Drosophila cellularization
Abstract
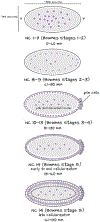
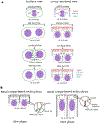
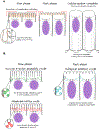
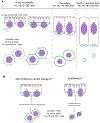
During morphogenesis, changes in the shapes of individual cells are harnessed to mold an entire tissue. These changes in cell shapes require the coupled remodeling of the plasma membrane and underlying actin cytoskeleton. In this review, we highlight cellularization of the Drosophila embryo as a model system to uncover principles of how membrane and actin dynamics are co-regulated in space and time to drive morphogenesis.
Keywords: Actin; Cortical compartments; Cytokinesis; Endocytosis; Exocytosis; Membrane reservoir; Membrane trafficking; Myosin-2; Phosphoinositides.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of Interest The authors declare that they have no conflicts of interest associated with this article.
Figures
Similar articles
-
Reshaping the Syncytial Drosophila Embryo with Cortical Actin Networks: Four Main Steps of Early Development.Results Probl Cell Differ. 2024;71:67-90. doi: 10.1007/978-3-031-37936-9_4. Results Probl Cell Differ. 2024. PMID: 37996673 Review.
-
Local actin-dependent endocytosis is zygotically controlled to initiate Drosophila cellularization.Dev Cell. 2008 May;14(5):775-86. doi: 10.1016/j.devcel.2008.02.014. Dev Cell. 2008. PMID: 18477459 Free PMC article.
-
Membrane Supply and Demand Regulates F-Actin in a Cell Surface Reservoir.Dev Cell. 2016 May 9;37(3):267-78. doi: 10.1016/j.devcel.2016.04.010. Dev Cell. 2016. PMID: 27165556 Free PMC article.
-
Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos.Mol Biol Cell. 2004 Feb;15(2):838-50. doi: 10.1091/mbc.e03-06-0440. Epub 2003 Dec 2. Mol Biol Cell. 2004. PMID: 14657248 Free PMC article.
-
Orchestrating morphogenesis: building the body plan by cell shape changes and movements.Development. 2020 Sep 11;147(17):dev191049. doi: 10.1242/dev.191049. Development. 2020. PMID: 32917667 Free PMC article. Review.
Cited by
-
Meiotic Cytokinesis in Saccharomyces cerevisiae: Spores That Just Need Closure.J Fungi (Basel). 2024 Feb 6;10(2):132. doi: 10.3390/jof10020132. J Fungi (Basel). 2024. PMID: 38392804 Free PMC article. Review.
-
Imaginal disk growth factors are Drosophila chitinase-like proteins with roles in morphogenesis and CO2 response.Genetics. 2023 Feb 9;223(2):iyac185. doi: 10.1093/genetics/iyac185. Genetics. 2023. PMID: 36576887 Free PMC article.
-
Early zygotic gene product Dunk interacts with anillin to regulate Myosin II during Drosophila cleavage.Mol Biol Cell. 2023 Sep 1;34(10):ar102. doi: 10.1091/mbc.E22-02-0046. Epub 2023 Jul 26. Mol Biol Cell. 2023. PMID: 37494082 Free PMC article.
-
Reshaping the Syncytial Drosophila Embryo with Cortical Actin Networks: Four Main Steps of Early Development.Results Probl Cell Differ. 2024;71:67-90. doi: 10.1007/978-3-031-37936-9_4. Results Probl Cell Differ. 2024. PMID: 37996673 Review.
-
Microtubule-Associated Serine/Threonine (MAST) Kinases in Development and Disease.Int J Mol Sci. 2023 Jul 25;24(15):11913. doi: 10.3390/ijms241511913. Int J Mol Sci. 2023. PMID: 37569286 Free PMC article. Review.
References
-
- Chugh P, Paluch EK, The actin cortex at a glance, J Cell Sci 131(14) (2018) https://www.ncbi.nlm.nih.gov/pubmed/30026344. - PMC - PubMed
-
- Kelkar M, Bohec P, Charras G, Mechanics of the cellular actin cortex: From signalling to shape change, Curr Opin Cell Biol 66 (2020) 69–78, https://www.ncbi.nlm.nih.gov/pubmed/32580115. - PubMed
-
- Mazumdar A, Mazumdar M, How one becomes many: blastoderm cellularization in Drosophila melanogaster, Bioessays 24(11) (2002) 1012–22, https://www.ncbi.nlm.nih.gov/pubmed/12386932. - PubMed
-
- Handel K, Grunfelder CG, Roth S, Sander K, Tribolium embryogenesis: a SEM study of cell shapes and movements from blastoderm to serosal closure, Dev Genes Evol 210(4) (2000) 167–79, https://www.ncbi.nlm.nih.gov/pubmed/11180819. - PubMed
-
- Kanayama M, Akiyama-Oda Y, Oda H, Early embryonic development in the spider Achaearanea tepidariorum: Microinjection verifies that cellularization is complete before the blastoderm stage, Arthropod Struct Dev 39(6) (2010) 436–45, https://www.ncbi.nlm.nih.gov/pubmed/20601115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

