Load adaptation by endocytic actin networks
- PMID: 35389747
- PMCID: PMC9265150
- DOI: 10.1091/mbc.E21-11-0589
Load adaptation by endocytic actin networks
Abstract
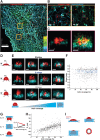
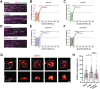
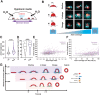
Clathrin-mediated endocytosis (CME) robustness under elevated membrane tension is maintained by actin assembly-mediated force generation. However, whether more actin assembles at endocytic sites in response to increased load has not previously been investigated. Here actin network ultrastructure at CME sites was examined under low and high membrane tension. Actin and N-WASP spatial organization indicate that actin polymerization initiates at the base of clathrin-coated pits and that the network then grows away from the plasma membrane. Actin network height at individual CME sites was not coupled to coat shape, raising the possibility that local differences in mechanical load feed back on assembly. By manipulating membrane tension and Arp2/3 complex activity, we tested the hypothesis that actin assembly at CME sites increases in response to elevated load. Indeed, in response to elevated membrane tension, actin grew higher, resulting in greater coverage of the clathrin coat, and CME slowed. When membrane tension was elevated and the Arp2/3 complex was inhibited, shallow clathrin-coated pits accumulated, indicating that this adaptive mechanism is especially crucial for coat curvature generation. We propose that actin assembly increases in response to increased load to ensure CME robustness over a range of plasma membrane tensions.
Figures

Similar articles
-
Principles of self-organization and load adaptation by the actin cytoskeleton during clathrin-mediated endocytosis.Elife. 2020 Jan 17;9:e49840. doi: 10.7554/eLife.49840. Elife. 2020. PMID: 31951196 Free PMC article.
-
Branched actin networks are organized for asymmetric force production during clathrin-mediated endocytosis in mammalian cells.Nat Commun. 2022 Jun 22;13(1):3578. doi: 10.1038/s41467-022-31207-5. Nat Commun. 2022. PMID: 35732852 Free PMC article.
-
N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits.J Cell Sci. 2005 Jul 15;118(Pt 14):3103-15. doi: 10.1242/jcs.02444. Epub 2005 Jun 28. J Cell Sci. 2005. PMID: 15985465
-
Local actin polymerization during endocytic carrier formation.Biochem Soc Trans. 2018 Jun 19;46(3):565-576. doi: 10.1042/BST20170355. Epub 2018 Apr 20. Biochem Soc Trans. 2018. PMID: 29678956 Review.
-
Clathrin coated pits, plaques and adhesion.J Struct Biol. 2016 Oct;196(1):48-56. doi: 10.1016/j.jsb.2016.07.009. Epub 2016 Jul 16. J Struct Biol. 2016. PMID: 27431447 Review.
Cited by
-
Mechanistic insights into actin force generation during vesicle formation from cryo-electron tomography.Dev Cell. 2022 May 9;57(9):1132-1145.e5. doi: 10.1016/j.devcel.2022.04.012. Epub 2022 May 2. Dev Cell. 2022. PMID: 35504288 Free PMC article.
-
Biochemical and mechanical regulation of actin dynamics.Nat Rev Mol Cell Biol. 2022 Dec;23(12):836-852. doi: 10.1038/s41580-022-00508-4. Epub 2022 Aug 2. Nat Rev Mol Cell Biol. 2022. PMID: 35918536 Review.
-
Clathrin coats partially preassemble and subsequently bend during endocytosis.J Cell Biol. 2023 Mar 6;222(3):e202206038. doi: 10.1083/jcb.202206038. Epub 2023 Feb 3. J Cell Biol. 2023. PMID: 36734980 Free PMC article.
-
Theoretical model of membrane protrusions driven by curved active proteins.Front Mol Biosci. 2023 May 9;10:1153420. doi: 10.3389/fmolb.2023.1153420. eCollection 2023. Front Mol Biosci. 2023. PMID: 37228585 Free PMC article.
-
Cellular and structural insight into dynamin function during endocytic vesicle formation: a tale of 50 years of investigation.Biosci Rep. 2022 Nov 30;42(11):BSR20211227. doi: 10.1042/BSR20211227. Biosci Rep. 2022. PMID: 36156116 Free PMC article. Review.
References
-
- Avinoam O, Schorb M, Beese CJ, Briggs JAG, Kaksonen M (2015). ENDOCYTOSIS. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science 348, 1369–1372. - PubMed

