Science-Based Approach to Harmonize Contraception Recommendations in Clinical Trials and Pharmaceutical Labels
- PMID: 35388453
- PMCID: PMC10083981
- DOI: 10.1002/cpt.2602
Science-Based Approach to Harmonize Contraception Recommendations in Clinical Trials and Pharmaceutical Labels
Abstract
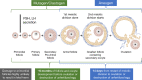
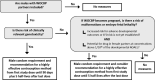
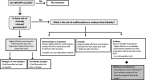
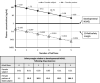
This review presents a European Federation of Pharmaceutical Industries and Association/PreClinical Development Expert Group (EFPIA-PDEG) topic group consensus on a data-driven approach to harmonized contraception recommendations for clinical trial protocols and product labeling. There is no international agreement in pharmaceutical clinical trial protocols or product labeling on when/if female and/or male contraception is warranted and for how long after the last dose. This absence of consensus has resulted in different recommendations among regions. For most pharmaceuticals, contraception recommendations are generally based exclusively on nonclinical data and/or mechanism. For clinical trials, contraception is the default position and is maintained for women throughout clinical development, whereas appropriate information can justify removing male contraception. Conversely, contraception is only recommended in product labeling when warranted. A base case rationale is proposed for whether or not female and/or male contraception is/are warranted, using available genotoxicity and developmental toxicity data. Contraception is generally warranted for both male and female subjects treated with mutagenic pharmaceuticals. We propose as a starting point that contraception is not typically warranted when the margin is 10-fold or greater between clinical exposure at the maximum recommended human dose and exposure at the no observed adverse effect level (NOAEL) for purely aneugenic pharmaceuticals and for pharmaceuticals that induce fetal malformations or embryo-fetal lethality. Other factors are discussed, including contraception methods, pregnancy testing, drug clearance, options for managing the absence of a developmental toxicity NOAEL, drug-drug interactions, radiopharmaceuticals, and other drug modalities. Overall, we present a data-driven rationale that can serve as a basis for consistent contraception recommendations in clinical trials and in product labeling across regions.
© 2022 Pfizer Inc. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employed by the pharmaceutical industry as stated in their respective affiliations.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Clinical trial considerations on male contraception and collection of pregnancy information from female partners.J Transl Med. 2012 Jun 21;10:129. doi: 10.1186/1479-5876-10-129. J Transl Med. 2012. PMID: 22720695 Free PMC article.
-
Canadian Contraception Consensus (Part 2 of 4).J Obstet Gynaecol Can. 2015 Nov;37(11):1033-9. doi: 10.1016/s1701-2163(16)30054-8. J Obstet Gynaecol Can. 2015. PMID: 26629725 English, French.
-
Canadian Contraception Consensus (Part 1 of 4).J Obstet Gynaecol Can. 2015 Oct;37(10):936-42. doi: 10.1016/s1701-2163(16)30033-0. J Obstet Gynaecol Can. 2015. PMID: 26606712 English, French.
-
Point of departure (PoD) selection for the derivation of acceptable daily exposures (ADEs) for active pharmaceutical ingredients (APIs).Regul Toxicol Pharmacol. 2016 Aug;79 Suppl 1:S48-56. doi: 10.1016/j.yrtph.2016.05.028. Epub 2016 May 24. Regul Toxicol Pharmacol. 2016. PMID: 27233925 Review.
Cited by
-
Responding to the Call to Action: Framework to Accelerate Clinical Data Generation for Antiretroviral Use in Pregnant Individuals with HIV.Infect Dis Ther. 2024 Jul;13(7):1391-1398. doi: 10.1007/s40121-024-00993-4. Epub 2024 May 22. Infect Dis Ther. 2024. PMID: 38772986 Free PMC article. No abstract available.
-
Pregnancy Outcomes in Patients Treated with Upadacitinib: Analysis of Data from Clinical Trials and Postmarketing Reports.Drug Saf. 2024 Oct;47(10):1039-1049. doi: 10.1007/s40264-024-01454-0. Epub 2024 Jul 15. Drug Saf. 2024. PMID: 39008024 Free PMC article.
References
-
- ICH . S2(R1) harmonised tripartite guideline. Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use <https://database.ich.org/sites/default/files/S2%28R1%29%20Guideline.pdf> (2011).
-
- ICH . S5(R3) Guidance on detection of reproductive and developmental toxicity for human pharmaceuticals <https://database.ich.org/sites/default/files/S5‐R3_Step4_Guideline_2020_...> (2020).
-
- ICH . M3(R2) Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals <https://database.ich.org/sites/default/files/M3_R2__Guideline.pdf> (2009). - PubMed
-
- Adam, M.P. , Polifka, J.E. & Friedman, J.M. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am. J. Med. Genet. C Semin. Med. Genet. 157C, 175–182 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

