Macrophage-derived IGF-1 protects the neonatal intestine against necrotizing enterocolitis by promoting microvascular development
- PMID: 35388142
- PMCID: PMC8987083
- DOI: 10.1038/s42003-022-03252-9
Macrophage-derived IGF-1 protects the neonatal intestine against necrotizing enterocolitis by promoting microvascular development
Abstract
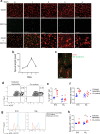
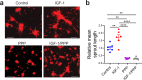
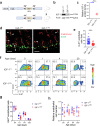
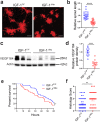
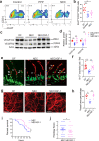
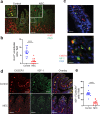
Necrotizing enterocolitis (NEC) is a deadly bowel necrotic disease of premature infants. Low levels of plasma IGF-1 predispose premature infants to NEC. While increasing evidence suggests that defective perinatal intestinal microvascular development plays a role in NEC, the involved mechanism remains incompletely understood. We report here that serum and intestinal IGF-1 are developmentally regulated during the perinatal period in mice and decrease during experimental NEC. Neonatal intestinal macrophages produce IGF-1 and promote endothelial cell sprouting in vitro via IGF-1 signaling. In vivo, in the neonatal intestine, macrophage-derived IGF-1 promotes VEGF expression and endothelial cell proliferation and protects against experimental NEC. Exogenous IGF-1 preserves intestinal microvascular density and protects against experimental NEC. In human NEC tissues, villous endothelial cell proliferation and IGF-1- producing macrophages are decreased compared to controls. Together, our results suggest that defective IGF-1-production by neonatal macrophages impairs neonatal intestinal microvascular development and predisposes the intestine to necrotizing enterocolitis.
© 2022. The Author(s).
Conflict of interest statement
I.D.P. received a grant from Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies, for testing rhIGF-1/BP3 in experimental NEC. X.Y., E.M, Y.Y.Z., and X.D.T. have no competing interests to declare.
Figures
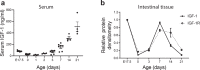


Similar articles
-
Recombinant IGF-1/BP3 protects against intestinal injury in a neonatal mouse NEC model.Pediatr Res. 2024 Jun;95(7):1803-1811. doi: 10.1038/s41390-024-03069-8. Epub 2024 Feb 28. Pediatr Res. 2024. PMID: 38418592
-
Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis.Am J Physiol Gastrointest Liver Physiol. 2019 Jul 1;317(1):G57-G66. doi: 10.1152/ajpgi.00332.2018. Epub 2019 May 24. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31125264 Free PMC article.
-
Dimethyloxalylglycine preserves the intestinal microvasculature and protects against intestinal injury in a neonatal mouse NEC model: role of VEGF signaling.Pediatr Res. 2018 Feb;83(2):545-553. doi: 10.1038/pr.2017.219. Epub 2017 Oct 25. Pediatr Res. 2018. PMID: 29068435 Free PMC article.
-
Intestinal microcirculation and necrotizing enterocolitis: The vascular endothelial growth factor system.Semin Fetal Neonatal Med. 2018 Dec;23(6):411-415. doi: 10.1016/j.siny.2018.08.008. Epub 2018 Sep 6. Semin Fetal Neonatal Med. 2018. PMID: 30213591 Review.
-
Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis.Semin Pediatr Surg. 2005 Aug;14(3):167-74. doi: 10.1053/j.sempedsurg.2005.05.005. Semin Pediatr Surg. 2005. PMID: 16084404 Review.
Cited by
-
Necrotizing enterocolitis: Bench to bedside approaches and advancing our understanding of disease pathogenesis.Front Pediatr. 2023 Jan 11;10:1107404. doi: 10.3389/fped.2022.1107404. eCollection 2022. Front Pediatr. 2023. PMID: 36714655 Free PMC article. Review.
-
Biomaterials for immunomodulation in wound healing.Regen Biomater. 2024 Mar 27;11:rbae032. doi: 10.1093/rb/rbae032. eCollection 2024. Regen Biomater. 2024. PMID: 38779347 Free PMC article. Review.
-
Feeding mode influences dynamic gut microbiota signatures and affects susceptibility to anti-CD3 mAb-induced intestinal injury in neonatal mice.Am J Physiol Gastrointest Liver Physiol. 2022 Sep 1;323(3):G205-G218. doi: 10.1152/ajpgi.00337.2021. Epub 2022 Jul 12. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 35819158 Free PMC article.
-
State-of-the-art review and update of in vivo models of necrotizing enterocolitis.Front Pediatr. 2023 Apr 4;11:1161342. doi: 10.3389/fped.2023.1161342. eCollection 2023. Front Pediatr. 2023. PMID: 37082706 Free PMC article. Review.
-
Gut microbiota differences in five-year-old children that were born preterm with a history of necrotizing enterocolitis: A pilot trial.iScience. 2024 Jun 20;27(7):110325. doi: 10.1016/j.isci.2024.110325. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055941 Free PMC article.
References
-
- Patel AL, Panagos PG, Silvestri JM. Reducing incidence of necrotizing enterocolitis. Clin. Perinatol. 2017;44:683–700. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

