A Ca2+-Dependent Mechanism Boosting Glycolysis and OXPHOS by Activating Aralar-Malate-Aspartate Shuttle, upon Neuronal Stimulation
- PMID: 35387872
- PMCID: PMC9097769
- DOI: 10.1523/JNEUROSCI.1463-21.2022
A Ca2+-Dependent Mechanism Boosting Glycolysis and OXPHOS by Activating Aralar-Malate-Aspartate Shuttle, upon Neuronal Stimulation
Abstract
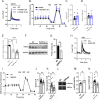
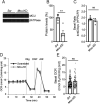
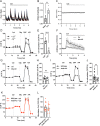
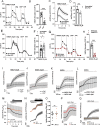
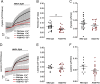
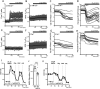
Calcium is an important second messenger regulating a bioenergetic response to the workloads triggered by neuronal activation. In embryonic mouse cortical neurons using glucose as only fuel, activation by NMDA elicits a strong workload (ATP demand)-dependent on Na+ and Ca2+ entry, and stimulates glucose uptake, glycolysis, pyruvate and lactate production, and oxidative phosphorylation (OXPHOS) in a Ca2+-dependent way. We find that Ca2+ upregulation of glycolysis, pyruvate levels, and respiration, but not glucose uptake, all depend on Aralar/AGC1/Slc25a12, the mitochondrial aspartate-glutamate carrier, component of the malate-aspartate shuttle (MAS). MAS activation increases glycolysis, pyruvate production, and respiration, a process inhibited in the presence of BAPTA-AM, suggesting that the Ca2+ binding motifs in Aralar may be involved in the activation. Mitochondrial calcium uniporter (MCU) silencing had no effect, indicating that none of these processes required MCU-dependent mitochondrial Ca2+ uptake. The neuronal respiratory response to carbachol was also dependent on Aralar, but not on MCU. We find that mouse cortical neurons are endowed with a constitutive ER-to-mitochondria Ca2+ flow maintaining basal cell bioenergetics in which ryanodine receptors, RyR2, rather than InsP3R, are responsible for Ca2+ release, and in which MCU does not participate. The results reveal that, in neurons using glucose, MCU does not participate in OXPHOS regulation under basal or stimulated conditions, while Aralar-MAS appears as the major Ca2+-dependent pathway tuning simultaneously glycolysis and OXPHOS to neuronal activation.SIGNIFICANCE STATEMENT Neuronal activation increases cell workload to restore ion gradients altered by activation. Ca2+ is involved in matching increased workload with ATP production, but the mechanisms are still unknown. We find that glycolysis, pyruvate production, and neuronal respiration are stimulated on neuronal activation in a Ca2+-dependent way, independently of effects of Ca2+ as workload inducer. Mitochondrial calcium uniporter (MCU) does not play a relevant role in Ca2+ stimulated pyruvate production and oxygen consumption as both are unchanged in MCU silenced neurons. However, Ca2+ stimulation is blunt in the absence of Aralar, a Ca2+-binding mitochondrial carrier component of Malate-Aspartate Shuttle (MAS). The results suggest that Ca2+-regulated Aralar-MAS activation upregulates glycolysis and pyruvate production, which fuels mitochondrial respiration, through regulation of cytosolic NAD+/NADH ratio.
Keywords: Aralar/AGC1/Slc25a12; calcium regulation; glycolysis; malate aspartate shuttle; mitochondrial calcium uniporter; neuronal metabolism.
Copyright © 2022 the authors.
Figures

Similar articles
-
Regulation of neuronal energy metabolism by calcium: Role of MCU and Aralar/malate-aspartate shuttle.Biochim Biophys Acta Mol Cell Res. 2023 Jun;1870(5):119468. doi: 10.1016/j.bbamcr.2023.119468. Epub 2023 Mar 28. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36997074
-
Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons.J Neurosci. 2013 Aug 28;33(35):13957-71, 13971a. doi: 10.1523/JNEUROSCI.0929-13.2013. J Neurosci. 2013. PMID: 23986233 Free PMC article.
-
Ca(2+) regulation of mitochondrial function in neurons.Biochim Biophys Acta. 2014 Oct;1837(10):1617-24. doi: 10.1016/j.bbabio.2014.04.010. Epub 2014 May 10. Biochim Biophys Acta. 2014. PMID: 24820519
-
Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
-
The regulation of neuronal mitochondrial metabolism by calcium.J Physiol. 2015 Aug 15;593(16):3447-62. doi: 10.1113/JP270254. J Physiol. 2015. PMID: 25809592 Free PMC article. Review.
Cited by
-
The New Nano-Resuscitation Solution (TPP-MR) Attenuated Myocardial Injury in Hemorrhagic Shock Rats by Inhibiting Ferroptosis.Int J Nanomedicine. 2024 Jul 25;19:7567-7583. doi: 10.2147/IJN.S463121. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39081897 Free PMC article.
-
The mitochondrial Ca2+ channel MCU is critical for tumor growth by supporting cell cycle progression and proliferation.Front Cell Dev Biol. 2023 Jun 8;11:1082213. doi: 10.3389/fcell.2023.1082213. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37363724 Free PMC article.
-
Mitochondrial regulation of local supply of energy in neurons.Curr Opin Neurobiol. 2023 Aug;81:102747. doi: 10.1016/j.conb.2023.102747. Epub 2023 Jun 29. Curr Opin Neurobiol. 2023. PMID: 37392672 Free PMC article. Review.
-
Mitochondrial VDAC1: A Potential Therapeutic Target of Inflammation-Related Diseases and Clinical Opportunities.Cells. 2022 Oct 10;11(19):3174. doi: 10.3390/cells11193174. Cells. 2022. PMID: 36231136 Free PMC article. Review.
-
Effects of Malate Ringer's solution on myocardial injury in sepsis and enforcement effects of TPP@PAMAM-MR.J Transl Med. 2022 Dec 13;20(1):591. doi: 10.1186/s12967-022-03811-y. J Transl Med. 2022. PMID: 36514103 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
