Pericytes' Circadian Clock Affects Endothelial Cells' Synchronization and Angiogenesis in a 3D Tissue Engineered Scaffold
- PMID: 35387328
- PMCID: PMC8977840
- DOI: 10.3389/fphar.2022.867070
Pericytes' Circadian Clock Affects Endothelial Cells' Synchronization and Angiogenesis in a 3D Tissue Engineered Scaffold
Abstract
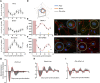
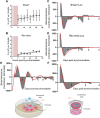
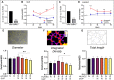
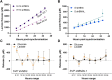
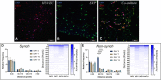
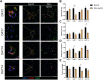
Angiogenesis, the formation of new capillaries from existing ones, is a fundamental process in regenerative medicine and tissue engineering. While it is known to be affected by circadian rhythms in vivo, its peripheral regulation within the vasculature and the role it performs in regulating the interplay between vascular cells have not yet been investigated. Peripheral clocks within the vasculature have been described in the endothelium and in smooth muscle cells. However, to date, scarce evidence has been presented regarding pericytes, a perivascular cell population deeply involved in the regulation of angiogenesis and vessel maturation, as well as endothelial function and homeostasis. More crucially, pericytes are also a promising source of cells for cell therapy and tissue engineering. Here, we established that human primary pericytes express key circadian genes and proteins in a rhythmic fashion upon synchronization. Conversely, we did not detect the same patterns in cultured endothelial cells. In line with these results, pericytes' viability was disproportionately affected by circadian cycle disruption, as compared to endothelial cells. Interestingly, endothelial cells' rhythm could be induced following exposure to synchronized pericytes in a contact co-culture. We propose that this mechanism could be linked to the altered release/uptake pattern of lactate, a known mediator of cell-cell interaction which was specifically altered in pericytes by the knockout of the key circadian regulator Bmal1. In an angiogenesis assay, the maturation of vessel-like structures was affected only when both endothelial cells and pericytes did not express Bmal1, indicating a compensation system. In a 3D tissue engineering scaffold, a synchronized clock supported a more structured organization of cells around the scaffold pores, and a maturation of vascular structures. Our results demonstrate that pericytes play a critical role in regulating the circadian rhythms in endothelial cells, and that silencing this system disproportionately affects their pro-angiogenic function. Particularly, in the context of tissue engineering and regenerative medicine, considering the effect of circadian rhythms may be critical for the development of mature vascular structures and to obtain the maximal reparative effect.
Keywords: angiogenesis; circadian; pericytes; tissue engineering and regenerative medicine; vasculature.
Copyright © 2022 Mastrullo, van der Veen, Gupta, Matos, Johnston, McVey, Madeddu, Velliou and Campagnolo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors.Biomaterials. 2013 Dec;34(36):9201-9. doi: 10.1016/j.biomaterials.2013.08.007. Epub 2013 Aug 22. Biomaterials. 2013. PMID: 23972477 Free PMC article.
-
Adverse Effect of Circadian Rhythm Disorder on Reparative Angiogenesis in Hind Limb Ischemia.J Am Heart Assoc. 2021 Aug 17;10(16):e020896. doi: 10.1161/JAHA.121.020896. Epub 2021 Aug 5. J Am Heart Assoc. 2021. PMID: 34348468 Free PMC article.
-
The circadian protein BMAL1 supports endothelial cell cycle during angiogenesis.Cardiovasc Res. 2023 Aug 19;119(10):1952-1968. doi: 10.1093/cvr/cvad057. Cardiovasc Res. 2023. PMID: 37052172
-
Pericytes in Tissue Engineering.Adv Exp Med Biol. 2018;1109:125-137. doi: 10.1007/978-3-030-02601-1_10. Adv Exp Med Biol. 2018. PMID: 30523594 Review.
-
Pericytes: Properties, Functions and Applications in Tissue Engineering.Stem Cell Rev Rep. 2015 Aug;11(4):549-59. doi: 10.1007/s12015-015-9590-z. Stem Cell Rev Rep. 2015. PMID: 25865146 Review.
Cited by
-
Development of circadian neurovascular function and its implications.Front Neurosci. 2023 Sep 5;17:1196606. doi: 10.3389/fnins.2023.1196606. eCollection 2023. Front Neurosci. 2023. PMID: 37732312 Free PMC article. Review.
-
Circadian Rhythms of the Blood-Brain Barrier and Drug Delivery.Circ Res. 2024 Mar 15;134(6):727-747. doi: 10.1161/CIRCRESAHA.123.323521. Epub 2024 Mar 14. Circ Res. 2024. PMID: 38484027 Review.
-
Temperature synchronisation of circadian rhythms in primary human airway epithelial cells from children.BMJ Open Respir Res. 2022 Oct;9(1):e001319. doi: 10.1136/bmjresp-2022-001319. BMJ Open Respir Res. 2022. PMID: 36198442 Free PMC article.
-
Hypertension: Causes and Consequences of Circadian Rhythms in Blood Pressure.Circ Res. 2024 Mar 15;134(6):810-832. doi: 10.1161/CIRCRESAHA.124.323515. Epub 2024 Mar 14. Circ Res. 2024. PMID: 38484034 Review.
-
The role of cardiac pericytes in health and disease: therapeutic targets for myocardial infarction.Nat Rev Cardiol. 2024 Feb;21(2):106-118. doi: 10.1038/s41569-023-00913-y. Epub 2023 Aug 4. Nat Rev Cardiol. 2024. PMID: 37542118 Review.
References
-
- Alvino V. V., Fernández-Jiménez R., Rodriguez-Arabaolaza I., Slater S., Mangialardi G., Avolio E., et al. (2018). Transplantation of Allogeneic Pericytes Improves Myocardial Vascularization and Reduces Interstitial Fibrosis in a Swine Model of Reperfused Acute Myocardial Infarction. J. Am. Heart Assoc. 7 (2), e006727. 10.1161/JAHA.117.006727 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

