Interrupting the Conversation: Implications for Crosstalk Between Viral and Bacterial Infections in the Asthmatic Airway
- PMID: 35386999
- PMCID: PMC8974750
- DOI: 10.3389/falgy.2021.738987
Interrupting the Conversation: Implications for Crosstalk Between Viral and Bacterial Infections in the Asthmatic Airway
Abstract
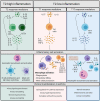
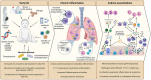
Asthma is a heterogeneous, chronic respiratory disease affecting 300 million people and is thought to be driven by different inflammatory endotypes influenced by a myriad of genetic and environmental factors. The complexity of asthma has rendered it challenging to develop preventative and disease modifying therapies and it remains an unmet clinical need. Whilst many factors have been implicated in asthma pathogenesis and exacerbations, evidence indicates a prominent role for respiratory viruses. However, advances in culture-independent detection methods and extensive microbial profiling of the lung, have also demonstrated a role for respiratory bacteria in asthma. In particular, airway colonization by the Proteobacteria species Nontypeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) is associated with increased risk of developing recurrent wheeze and asthma in early life, poor clinical outcomes in established adult asthma and the development of more severe inflammatory phenotypes. Furthermore, emerging evidence indicates that bacterial-viral interactions may influence exacerbation risk and disease severity, highlighting the need to consider the impact chronic airway colonization by respiratory bacteria has on influencing host responses to viral infection. In this review, we first outline the currently understood role of viral and bacterial infections in precipitating asthma exacerbations and discuss the underappreciated potential impact of bacteria-virus crosstalk in modulating host responses. We discuss the mechanisms by which early life infection may predispose to asthma development. Finally, we consider how infection and persistent airway colonization may drive different asthma phenotypes, with a view to identifying pathophysiological mechanisms that may prove tractable to new treatment modalities.
Keywords: asthma; bacteria; co-infection; early-life; exacerbation; inflammation; virus.
Copyright © 2021 Ackland, Watson, Wilkinson and Staples.
Conflict of interest statement
KS reports grants from AstraZeneca outside the conduct of the study. TW reports grants and personal fees from AstraZeneca, personal fees and other from MMH, grants and personal fees from GSK, grants and personal fees from AZ, personal fees from BI, grants and personal fees from Synairgen, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Childhood asthma after bacterial colonization of the airway in neonates.N Engl J Med. 2007 Oct 11;357(15):1487-95. doi: 10.1056/NEJMoa052632. N Engl J Med. 2007. PMID: 17928596
-
The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae.Front Immunol. 2018 Nov 5;9:2530. doi: 10.3389/fimmu.2018.02530. eCollection 2018. Front Immunol. 2018. PMID: 30455693 Free PMC article. Review.
-
The role of NTHi colonization and infection in the pathogenesis of neutrophilic asthma.Respir Res. 2020 Jul 3;21(1):170. doi: 10.1186/s12931-020-01438-5. Respir Res. 2020. PMID: 32620122 Free PMC article. Review.
-
Low-grade disease activity in early life precedes childhood asthma and allergy.Dan Med J. 2016 Aug;63(8):B5272. Dan Med J. 2016. PMID: 27477800 Review.
-
Viral Infections and Associated Factors That Promote Acute Exacerbations of Asthma.Allergy Asthma Immunol Res. 2018 Jan;10(1):12-17. doi: 10.4168/aair.2018.10.1.12. Allergy Asthma Immunol Res. 2018. PMID: 29178673 Free PMC article. Review.
Cited by
-
Nontypeable Haemophilus influenzae infection of pulmonary macrophages drives neutrophilic inflammation in severe asthma.Allergy. 2022 Oct;77(10):2961-2973. doi: 10.1111/all.15375. Epub 2022 May 30. Allergy. 2022. PMID: 35570583 Free PMC article.
-
Epidemiology and Immunopathogenesis of Virus Associated Asthma Exacerbations.J Asthma Allergy. 2023 Sep 26;16:1025-1040. doi: 10.2147/JAA.S277455. eCollection 2023. J Asthma Allergy. 2023. PMID: 37791040 Free PMC article. Review.
-
Characterization and inhibition of inflammasome responses in severe and non-severe asthma.Respir Res. 2023 Dec 4;24(1):303. doi: 10.1186/s12931-023-02603-2. Respir Res. 2023. PMID: 38044426 Free PMC article.
-
Impact of Therapeutics on Unified Immunity During Allergic Asthma and Respiratory Infections.Front Allergy. 2022 Mar 25;3:852067. doi: 10.3389/falgy.2022.852067. eCollection 2022. Front Allergy. 2022. PMID: 35386652 Free PMC article. Review.
-
Moraxella occupied the largest proportion in the nasal microbiome in healthy children, which potential protect them from COVID-19.Microb Pathog. 2022 Sep;170:105685. doi: 10.1016/j.micpath.2022.105685. Epub 2022 Jul 21. Microb Pathog. 2022. PMID: 35870694 Free PMC article.
References
-
- Global Initiative for Asthma . Global Strategy For Asthma Management and Prevention (2021). Available online at: www.ginasthma.org
Publication types
LinkOut - more resources
Full Text Sources

