Polyphosphate-crosslinked collagen scaffolds for hemostasis and alveolar bone regeneration after tooth extraction
- PMID: 35386354
- PMCID: PMC8940764
- DOI: 10.1016/j.bioactmat.2021.12.019
Polyphosphate-crosslinked collagen scaffolds for hemostasis and alveolar bone regeneration after tooth extraction
Abstract
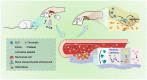
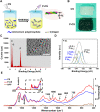
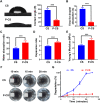
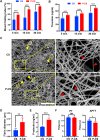
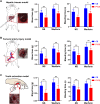
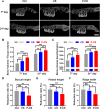
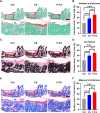
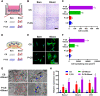
Post-extraction bleeding and alveolar bone resorption are the two frequently encountered complications after tooth extraction that result in poor healing and rehabilitation difficulties. The present study covalently bonded polyphosphate onto a collagen scaffold (P-CS) by crosslinking. The P-CS demonstrated improved hemostatic property in a healthy rat model and an anticoagulant-treated rat model. This improvement is attributed to the increase in hydrophilicity, increased thrombin generation, platelet activation and stimulation of the intrinsic coagulation pathway. In addition, the P-CS promoted the in-situ bone regeneration and alveolar ridge preservation in a rat alveolar bone defect model. The promotion is attributed to enhanced osteogenic differentiation of bone marrow stromal cells. Osteogenesis was improved by both polyphosphate and blood clots. Taken together, P-CS possesses favorable hemostasis and alveolar ridge preservation capability. It may be used as an effective treatment option for post-extraction bleeding and alveolar bone loss.
Statement of significance: Collagen scaffold is commonly used for the treatment of post-extraction bleeding and alveolar bone loss after tooth extraction. However, its application is hampered by insufficient hemostatic and osteoinductive property. Crosslinking polyphosphate with collagen produces a modified collagen scaffold that possesses improved hemostatic performance and augmented bone regeneration potential.
Keywords: Alveolar ridge preservation; Blood clotting; Osteogenesis; Polyphosphate.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
An injectable and coagulation-independent Tetra-PEG hydrogel bioadhesive for post-extraction hemostasis and alveolar bone regeneration.Bioact Mater. 2024 Mar 19;37:106-118. doi: 10.1016/j.bioactmat.2024.03.015. eCollection 2024 Jul. Bioact Mater. 2024. PMID: 39022616 Free PMC article.
-
Shape memory and hemostatic silk-laponite scaffold for alveolar bone regeneration after tooth extraction trauma.Int J Biol Macromol. 2024 Mar;260(Pt 2):129454. doi: 10.1016/j.ijbiomac.2024.129454. Epub 2024 Jan 16. Int J Biol Macromol. 2024. PMID: 38237836
-
Nano-hydroxyapatite mineralized silk fibroin porous scaffold for tooth extraction site preservation.Dent Mater. 2019 Oct;35(10):1397-1407. doi: 10.1016/j.dental.2019.07.024. Epub 2019 Aug 6. Dent Mater. 2019. PMID: 31395452
-
Ridge preservation after tooth extraction.Clin Oral Implants Res. 2012 Oct;23 Suppl 6:147-56. doi: 10.1111/j.1600-0501.2012.02560.x. Clin Oral Implants Res. 2012. PMID: 23062139 Review.
-
[Prevention of alveolar ridge resorption after tooth extraction--a review].Schweiz Monatsschr Zahnmed. 2004;114(4):328-36. Schweiz Monatsschr Zahnmed. 2004. PMID: 15185481 Review. German.
Cited by
-
An injectable and coagulation-independent Tetra-PEG hydrogel bioadhesive for post-extraction hemostasis and alveolar bone regeneration.Bioact Mater. 2024 Mar 19;37:106-118. doi: 10.1016/j.bioactmat.2024.03.015. eCollection 2024 Jul. Bioact Mater. 2024. PMID: 39022616 Free PMC article.
-
Biopolymers and Their Application in Bioprinting Processes for Dental Tissue Engineering.Pharmaceutics. 2023 Aug 10;15(8):2118. doi: 10.3390/pharmaceutics15082118. Pharmaceutics. 2023. PMID: 37631331 Free PMC article. Review.
-
Macro, Micro, and Nano-Inspired Bioactive Polymeric Biomaterials in Therapeutic, and Regenerative Orofacial Applications.Drug Des Devel Ther. 2023 Sep 27;17:2985-3021. doi: 10.2147/DDDT.S419361. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37789970 Free PMC article. Review.
-
A Biodegradable Tissue Adhesive for Post-Extraction Alveolar Bone Regeneration under Ongoing Anticoagulation-A Microstructural Volumetric Analysis in a Rodent Model.Int J Mol Sci. 2024 Apr 10;25(8):4210. doi: 10.3390/ijms25084210. Int J Mol Sci. 2024. PMID: 38673796 Free PMC article.
-
Tissue-Engineered Nanomaterials Play Diverse Roles in Bone Injury Repair.Nanomaterials (Basel). 2023 Apr 24;13(9):1449. doi: 10.3390/nano13091449. Nanomaterials (Basel). 2023. PMID: 37176994 Free PMC article. Review.
References
-
- Krishanappa S., Hassan H. Interventions for treating post-extraction bleeding. Cochrane Database Syst. Rev. 2018;3 doi: 10.1002/14651858.CD011930.pub3.www.cochranelibrary.com. - DOI - PMC - PubMed
-
- Iwabuchi H., Imai Y., Asanami S., Shirakawa M., Yamane G., Ogiuchi H., Kurashina K., Miyata M., Nakao H., Imai H. Evaluation of postextraction bleeding incidence to compare patients receiving and not receiving warfarin therapy : observational study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005777. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

