Breast cancer metastasis to brain results in recruitment and activation of microglia through annexin-A1/formyl peptide receptor signaling
- PMID: 35382852
- PMCID: PMC8985313
- DOI: 10.1186/s13058-022-01514-2
Breast cancer metastasis to brain results in recruitment and activation of microglia through annexin-A1/formyl peptide receptor signaling
Abstract
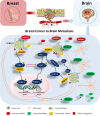
Background: Despite advancements in therapies, brain metastasis in patients with triple negative subtype of breast cancer remains a therapeutic challenge. Activated microglia are often observed in close proximity to, or within, malignant tumor masses, suggesting a critical role that microglia play in brain tumor progression. Annexin-A1 (ANXA1), a glucocorticoid-regulated protein with immune-regulatory properties, has been implicated in the growth and metastasis of many cancers. Its role in breast cancer-microglia signaling crosstalk is not known.
Methods: The importance of microglia proliferation and activation in breast cancer to brain metastasis was evaluated in MMTV-Wnt1 spontaneous mammary tumor mice and BALBc mice injected with 4T1 murine breast cancer cells into the carotid artery using flow cytometry. 4T1 induced-proliferation and migration of primary microglia and BV2 microglia cells were evaluated using 2D and coculture transwell assays. The requirement of ANXA1 in these functions was examined using a Crispr/Cas9 deletion mutant of ANXA1 in 4T1 breast cancer cells as well as BV2 microglia. Small molecule inhibition of the ANXA1 receptor FPR1 and FPR2 were also examined. The signaling pathways involved in these interactions were assessed using western blotting. The association between lymph node positive recurrence-free patient survival and distant metastasis-free patient survival and ANXA1 and FPR1 and FPR2 expression was examined using TCGA datasets.
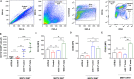
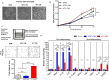
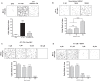
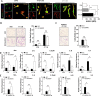
Results: Microglia activation is observed prior to brain metastasis in MMTV-Wnt1 mice with primary and secondary metastasis in the periphery. Metastatic 4T1 mammary cancer cells secrete ANXA1 to promote microglial migration, which in turn, enhances tumor cell migration. Silencing of ANXA1 in 4T1 cells by Crispr/Cas9 deletion, or using inhibitors of FPR1 or FPR2 inhibits microglia migration and leads to reduced activation of STAT3. Finally, elevated ANXA1, FPR1 and FPR2 is significantly associated with poor outcome in lymph node positive patients, particularly, for distant metastasis free patient survival.
Conclusions: The present study uncovered a network encompassing autocrine/paracrine ANXA1 signaling between metastatic mammary cancer cells and microglia that drives microglial recruitment and activation. Inhibition of ANXA1 and/or its receptor may be therapeutically rewarding in the treatment of breast cancer and secondary metastasis to the brain.
Keywords: Annexin-A1; Brain metastasis; Breast cancer; FPR2; Microglia; STAT3.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no other competing interests or conflicts of interest.
Figures



Similar articles
-
An overview of the regulatory role of annexin A1 in the tumor microenvironment and its prospective clinical application (Review).Int J Oncol. 2024 May;64(5):51. doi: 10.3892/ijo.2024.5639. Epub 2024 Mar 22. Int J Oncol. 2024. PMID: 38516766 Free PMC article. Review.
-
Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2.FASEB J. 2011 Feb;25(2):483-96. doi: 10.1096/fj.09-154096. Epub 2010 Oct 7. FASEB J. 2011. PMID: 20930115
-
Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma.PLoS One. 2014 Dec 9;9(12):e111317. doi: 10.1371/journal.pone.0111317. eCollection 2014. PLoS One. 2014. PMID: 25490767 Free PMC article.
-
Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo.Biochim Biophys Acta Mol Cell Res. 2018 Sep;1865(9):1368-1382. doi: 10.1016/j.bbamcr.2018.06.010. Epub 2018 Jun 20. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29932988
-
Annexin-A1: Therapeutic Potential in Microvascular Disease.Front Immunol. 2019 Apr 30;10:938. doi: 10.3389/fimmu.2019.00938. eCollection 2019. Front Immunol. 2019. PMID: 31114582 Free PMC article. Review.
Cited by
-
Blocking the MIF-CD74 axis augments radiotherapy efficacy for brain metastasis in NSCLC via synergistically promoting microglia M1 polarization.J Exp Clin Cancer Res. 2024 Apr 29;43(1):128. doi: 10.1186/s13046-024-03024-9. J Exp Clin Cancer Res. 2024. PMID: 38685050 Free PMC article.
-
An overview of the regulatory role of annexin A1 in the tumor microenvironment and its prospective clinical application (Review).Int J Oncol. 2024 May;64(5):51. doi: 10.3892/ijo.2024.5639. Epub 2024 Mar 22. Int J Oncol. 2024. PMID: 38516766 Free PMC article. Review.
-
The roles of tissue resident macrophages in health and cancer.Exp Hematol Oncol. 2024 Jan 16;13(1):3. doi: 10.1186/s40164-023-00469-0. Exp Hematol Oncol. 2024. PMID: 38229178 Free PMC article. Review.
-
Harnessing immunotherapy for brain metastases: insights into tumor-brain microenvironment interactions and emerging treatment modalities.J Hematol Oncol. 2023 Dec 16;16(1):121. doi: 10.1186/s13045-023-01518-1. J Hematol Oncol. 2023. PMID: 38104104 Free PMC article. Review.
-
Analysis of Dormancy-Associated Transcriptional Networks Reveals a Shared Quiescence Signature in Lung and Colorectal Cancer.Int J Mol Sci. 2022 Aug 30;23(17):9869. doi: 10.3390/ijms23179869. Int J Mol Sci. 2022. PMID: 36077264 Free PMC article.
References
-
- Apuri S. Neoadjuvant and adjuvant therapies for breast cancer. South Med J. 2017;110:638–642. - PubMed
-
- Corona SP, et al. Advances in systemic therapy for metastatic breast cancer: future perspectives. Med Oncol. 2017;34:119. - PubMed
-
- da Fonseca AC, et al. Microglia in cancer: For good or for bad? Adv Exp Med Biol. 2016;949:245–261. - PubMed
-
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

