Adipose tissue aging: mechanisms and therapeutic implications
- PMID: 35379822
- PMCID: PMC8980023
- DOI: 10.1038/s41419-022-04752-6
Adipose tissue aging: mechanisms and therapeutic implications
Abstract
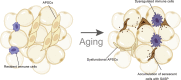
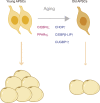
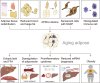
Adipose tissue, which is the crucial energy reservoir and endocrine organ for the maintenance of systemic glucose, lipid, and energy homeostasis, undergoes significant changes during aging. These changes cause physiological declines and age-related disease in the elderly population. Here, we review the age-related changes in adipose tissue at multiple levels and highlight the underlying mechanisms regulating the aging process. We also discuss the pathogenic pathways of age-related fat dysfunctions and their systemic negative consequences, such as dyslipidemia, chronic general inflammation, insulin resistance, and type 2 diabetes (T2D). Age-related changes in adipose tissue involve redistribution of deposits and composition, in parallel with the functional decline of adipocyte progenitors and accumulation of senescent cells. Multiple pathogenic pathways induce defective adipogenesis, inflammation, aberrant adipocytokine production, and insulin resistance, leading to adipose tissue dysfunction. Changes in gene expression and extracellular signaling molecules regulate the aging process of adipose tissue through various pathways. In addition, adipose tissue aging impacts other organs that are infiltrated by lipids, which leads to systemic inflammation, metabolic system disruption, and aging process acceleration. Moreover, studies have indicated that adipose aging is an early onset event in aging and a potential target to extend lifespan. Together, we suggest that adipose tissue plays a key role in the aging process and is a therapeutic target for the treatment of age-related disease, which deserves further study to advance relevant knowledge.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications.Int J Mol Sci. 2019 May 13;20(9):2358. doi: 10.3390/ijms20092358. Int J Mol Sci. 2019. PMID: 31085992 Free PMC article. Review.
-
Physiological Approaches Targeting Cellular and Mitochondrial Pathways Underlying Adipose Organ Senescence.Int J Mol Sci. 2023 Jul 19;24(14):11676. doi: 10.3390/ijms241411676. Int J Mol Sci. 2023. PMID: 37511435 Free PMC article. Review.
-
Recent advances in the effect of adipose tissue inflammation on insulin resistance.Cell Signal. 2024 Aug;120:111229. doi: 10.1016/j.cellsig.2024.111229. Epub 2024 May 17. Cell Signal. 2024. PMID: 38763181 Review.
-
Physiological Aging: Links Among Adipose Tissue Dysfunction, Diabetes, and Frailty.Physiology (Bethesda). 2017 Jan;32(1):9-19. doi: 10.1152/physiol.00012.2016. Physiology (Bethesda). 2017. PMID: 27927801 Free PMC article. Review.
-
The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders.Clin Sci (Lond). 2020 Jan 31;134(2):315-330. doi: 10.1042/CS20190966. Clin Sci (Lond). 2020. PMID: 31998947 Review.
Cited by
-
Considerations for long-acting antiretroviral therapy in older persons with HIV.AIDS. 2023 Dec 1;37(15):2271-2286. doi: 10.1097/QAD.0000000000003704. Epub 2023 Nov 16. AIDS. 2023. PMID: 37965737 Free PMC article. Review.
-
Inflammaging and fatty acid oxidation in monocytes and macrophages.Immunometabolism (Cobham). 2024 Jan 19;6(1):e00038. doi: 10.1097/IN9.0000000000000038. eCollection 2024 Jan. Immunometabolism (Cobham). 2024. PMID: 38249577 Free PMC article. Review.
-
Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight.Biomolecules. 2024 Sep 27;14(10):1223. doi: 10.3390/biom14101223. Biomolecules. 2024. PMID: 39456156 Free PMC article. Review.
-
The impact of the senescent microenvironment on tumorigenesis: Insights for cancer therapy.Aging Cell. 2024 May;23(5):e14182. doi: 10.1111/acel.14182. Epub 2024 Apr 22. Aging Cell. 2024. PMID: 38650467 Free PMC article. Review.
-
Biomarkers of aging.Sci China Life Sci. 2023 May;66(5):893-1066. doi: 10.1007/s11427-023-2305-0. Epub 2023 Apr 11. Sci China Life Sci. 2023. PMID: 37076725 Free PMC article. Review.
References
-
- Coin A, Sergi G, Inelmen EM, Enzi G. Pathophysiology of body composition changes in elderly people. In: Cachexia and wasting: a modern approach. Milano: Springer; 2006. p. 369–75.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

