Targeting lncRNAs in programmed cell death as a therapeutic strategy for non-small cell lung cancer
- PMID: 35379783
- PMCID: PMC8980082
- DOI: 10.1038/s41420-022-00982-x
Targeting lncRNAs in programmed cell death as a therapeutic strategy for non-small cell lung cancer
Abstract
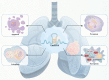
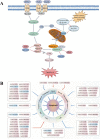
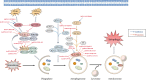
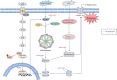
Lung cancer is a leading cause of cancer-related mortality worldwide, with non-small cell lung cancer (NSCLC) being the most common histological type. Owing to the limited therapeutic efficacy and side effects of currently available therapies for NSCLC, it is necessary to identify novel therapeutic targets for NSCLC. Long non-coding RNAs (lncRNAs) are non-protein-coding RNAs with a transcript length of more than 200 nucleotides, which play a vital role in the tumorigenesis and progression of multiple cancers, including NSCLC. Induction of programmed cell death (PCD) is the main mechanism leading to tumour cell death in most cancer treatments. Recent studies have demonstrated that lncRNAs are closely correlated with PCD including apoptosis, pyroptosis, autophagy and ferroptosis, which can regulate PCD and relevant death pathways to affect NSCLC progression and the efficacy of clinical therapy. Therefore, in this review, we focused on the function of lncRNAs in PCD of NSCLC and summarized the therapeutic role of targeting lncRNAs in PCD for NSCLC treatment, aiming to provide new sights into the underlying pathogenic mechanisms and propose a potential new strategy for NSCLC therapy so as to improve therapeutic outcomes with the ultimate goal to benefit the patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Regulation of apoptosis, autophagy and ferroptosis by non-coding RNAs in metastatic non-small cell lung cancer (Review).Exp Ther Med. 2022 May;23(5):352. doi: 10.3892/etm.2022.11279. Epub 2022 Mar 28. Exp Ther Med. 2022. PMID: 35493430 Free PMC article. Review.
-
Long non-coding RNAs: How to regulate the metastasis of non-small-cell lung cancer.J Cell Mol Med. 2020 Mar;24(6):3282-3291. doi: 10.1111/jcmm.15054. Epub 2020 Feb 12. J Cell Mol Med. 2020. PMID: 32048814 Free PMC article. Review.
-
Long noncoding RNAs: new insights into non-small cell lung cancer biology, diagnosis and therapy.Med Oncol. 2016 Feb;33(2):18. doi: 10.1007/s12032-016-0731-2. Epub 2016 Jan 19. Med Oncol. 2016. PMID: 26786153 Review.
-
LncRNAs in Non-Small-Cell Lung Cancer.Noncoding RNA. 2020 Jun 30;6(3):25. doi: 10.3390/ncrna6030025. Noncoding RNA. 2020. PMID: 32629922 Free PMC article. Review.
-
Progress in understanding the role of lncRNA in programmed cell death.Cell Death Discov. 2021 Feb 8;7(1):30. doi: 10.1038/s41420-021-00407-1. Cell Death Discov. 2021. PMID: 33558499 Free PMC article. Review.
Cited by
-
lncHUB2: aggregated and inferred knowledge about human and mouse lncRNAs.Database (Oxford). 2023 Mar 4;2023:baad009. doi: 10.1093/database/baad009. Database (Oxford). 2023. PMID: 36869839 Free PMC article.
-
Long noncoding RNA BBOX1-AS1 increased radiotherapy sensitivity in colorectal cancer by stabilizing and activating PFK1.Transl Oncol. 2023 Oct;36:101751. doi: 10.1016/j.tranon.2023.101751. Epub 2023 Aug 4. Transl Oncol. 2023. PMID: 37544035 Free PMC article.
-
[Role of Ferroptosis in Non-small Cell Lung Cancer and Progress of Traditional Chinese Medicine Intervention].Zhongguo Fei Ai Za Zhi. 2024 Mar 20;27(3):216-230. doi: 10.3779/j.issn.1009-3419.2024.101.06. Zhongguo Fei Ai Za Zhi. 2024. PMID: 38590196 Free PMC article. Review. Chinese.
-
Long noncoding RNA POU6F2-AS2 contributes to the aggressiveness of nonsmall-cell lung cancer via microRNA-125b-5p-mediated E2F3 upregulation.Aging (Albany NY). 2023 Apr 6;15(7):2689-2704. doi: 10.18632/aging.204639. Epub 2023 Apr 6. Aging (Albany NY). 2023. PMID: 37053020 Free PMC article.
-
SETD1A-mediated H3K4me3 methylation upregulates lncRNA HOXC-AS3 and the binding of HOXC-AS3 to EP300 and increases EP300 stability to suppress the ferroptosis of NSCLC cells.Thorac Cancer. 2023 Sep;14(25):2579-2590. doi: 10.1111/1759-7714.15037. Epub 2023 Aug 7. Thorac Cancer. 2023. PMID: 37548102 Free PMC article.
References
-
- Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157:103194. - PubMed
-
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–40. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

