Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial
- PMID: 35378077
- PMCID: PMC9077443
- DOI: 10.1016/S0140-6736(22)00538-4
Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial
Erratum in
-
Department of Error.Lancet. 2022 May 7;399(10337):1778. doi: 10.1016/S0140-6736(22)00783-8. Lancet. 2022. PMID: 35526551 Free PMC article. No abstract available.
Abstract
Background: Oral pre-exposure prophylaxis has been introduced in more than 70 countries, including many in sub-Saharan Africa, but women experience considerable barriers to daily pill-taking, such as stigma, judgement, and the fear of violence. Safe and effective long-acting agents for HIV prevention are needed for women. We aimed to evaluate the safety and efficacy of injectable cabotegravir compared with daily oral tenofovir diphosphate plus emtricitabine (TDF-FTC) for HIV prevention in HIV-uninfected women.
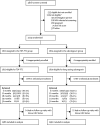
Methods: HPTN 084 was a phase 3, randomised, double-blind, double-dummy, active-controlled, superiority trial in 20 clinical research sites in seven countries in sub-Saharan Africa. Participants were eligible for enrolment if they were assigned female sex at birth, were aged 18-45 years, reported at least two episodes of vaginal intercourse in the previous 30 days, were at risk of HIV infection based on an HIV risk score, and agreed to use a long-acting reversible contraceptive method. Participants were randomly assigned (1:1) to either active cabotegravir with TDF-FTC placebo (cabotegravir group) or active TDF-FTC with cabotegravir placebo (TDF-FTC group). Study staff and participants were masked to study group allocation, with the exception of the site pharmacist who was responsible for study product preparation. Participants were prescribed 5 weeks of daily oral product followed by intramuscular injections every 8 weeks after an initial 4-week interval load, alongside daily oral pills. Participants who discontinued injections were offered open-label daily TDF-FTC for 48 weeks. The primary endpoints of the study were incident HIV infection in the intention-to-treat population, and clinical and laboratory events that were grade 2 or higher in all women who had received at least one dose of study product. This study is registered with ClinicalTrials.gov, NCT03164564.
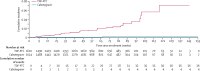
Findings: From Nov 27, 2017, to Nov 4, 2020, we enrolled 3224 participants (1614 in the cabotegravir group and 1610 in the TDF-FTC group). Median age was 25 years (IQR 22-30); 1755 (54·7%) of 3209 had two or more partners in the preceding month. 40 incident infections were observed over 3898 person-years (HIV incidence 1·0% [95% CI 0·73-1·40]); four in the cabotegravir group (HIV incidence 0·2 cases per 100 person-years [0·06-0·52]) and 36 in the TDF-FTC group (1·85 cases per 100 person-years [1·3-2·57]; hazard ratio 0·12 [0·05-0·31]; p<0·0001; risk difference -1·6% [-1·0% to -2·3%]. In a random subset of 405 TDF-FTC participants, 812 (42·1%) of 1929 plasma samples had tenofovir concentrations consistent with daily use. Injection coverage was 93% of the total number of person-years. Adverse event rates were similar across both groups, apart from injection site reactions, which were more frequent in the cabotegravir group than in the TDF-FTC group (577 [38·0%] of 1519 vs 162 [10·7%] of 1516]) but did not result in injection discontinuation. Confirmed pregnancy incidence was 1·3 per 100 person-years (0·9-1·7); no congenital birth anomalies were reported.
Interpretation: Although both products for HIV prevention were generally safe, well tolerated, and effective, cabotegravir was superior to TDF-FTC in preventing HIV infection in women.
Funding: National Institute of Allergy and Infectious Diseases, ViiV Healthcare, and the Bill & Melinda Gates Foundation. Additional support was provided through the National Institute of Mental Health, the National Institute on Drug Abuse, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. ViiV Healthcare and Gilead Sciences provided pharmaceutical support.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests PLA has received fees from Merck, ViiV Healthcare, and Gilead Sciences, and research support from Gilead Sciences paid to his institution. AR, WRS, and KS are employees of ViiV Healthcare. JFR is an employee of Gilead Sciences. All other authors declare no competing interests.
Figures


Comment in
-
Long-acting injections for HIV prevention among women in sub-Saharan Africa.Lancet. 2022 May 7;399(10337):1754-1755. doi: 10.1016/S0140-6736(22)00613-4. Epub 2022 Apr 1. Lancet. 2022. PMID: 35378079 No abstract available.
-
Long-acting agents for HIV treatment and prevention.Nat Med. 2022 Aug;28(8):1542-1543. doi: 10.1038/s41591-022-01917-w. Nat Med. 2022. PMID: 35869309 No abstract available.
-
Long-lasting HIV prevention drug could be game changer - but who will pay?Nature. 2022 Aug;608(7923):460-461. doi: 10.1038/d41586-022-02123-x. Nature. 2022. PMID: 35931763 No abstract available.
-
Preventive HIV drug shows urgent need for transparency on pricing.Nature. 2022 Aug;608(7922):239. doi: 10.1038/d41586-022-02136-6. Nature. 2022. PMID: 35945377 No abstract available.
Similar articles
-
Efficacy and safety of long-acting cabotegravir compared with daily oral tenofovir disoproxil fumarate plus emtricitabine to prevent HIV infection in cisgender men and transgender women who have sex with men 1 year after study unblinding: a secondary analysis of the phase 2b and 3 HPTN 083 randomised controlled trial.Lancet HIV. 2023 Dec;10(12):e767-e778. doi: 10.1016/S2352-3018(23)00261-8. Epub 2023 Nov 9. Lancet HIV. 2023. PMID: 37952550 Free PMC article. Clinical Trial.
-
Evaluation of long-acting cabotegravir safety and pharmacokinetics in pregnant women in eastern and southern Africa: a secondary analysis of HPTN 084.J Int AIDS Soc. 2025 Jan;28(1):e26401. doi: 10.1002/jia2.26401. J Int AIDS Soc. 2025. PMID: 39748218 Free PMC article. Clinical Trial.
-
Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women.N Engl J Med. 2021 Aug 12;385(7):595-608. doi: 10.1056/NEJMoa2101016. N Engl J Med. 2021. PMID: 34379922 Free PMC article. Clinical Trial.
-
Oxycodone for neuropathic pain and fibromyalgia in adults.Cochrane Database Syst Rev. 2014 Jun 23;(6):CD010692. doi: 10.1002/14651858.CD010692.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Jul 28;7:CD010692. doi: 10.1002/14651858.CD010692.pub3. PMID: 24956205 Updated. Review.
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article. Review.
Cited by
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
HIV Preexposure Prophylaxis With Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women.JAMA. 2024 Mar 19;331(11):930-937. doi: 10.1001/jama.2024.0464. JAMA. 2024. PMID: 38427359 Free PMC article.
-
High PrEP uptake and objective longitudinal adherence among HIV-exposed women with personal or partner plans for pregnancy in rural Uganda: A cohort study.PLoS Med. 2023 Feb 16;20(2):e1004088. doi: 10.1371/journal.pmed.1004088. eCollection 2023 Feb. PLoS Med. 2023. PMID: 36795763 Free PMC article.
-
Predicted effects of the introduction of long-acting injectable cabotegravir pre-exposure prophylaxis in sub-Saharan Africa: a modelling study.Lancet HIV. 2023 Apr;10(4):e254-e265. doi: 10.1016/S2352-3018(22)00365-4. Epub 2023 Jan 12. Lancet HIV. 2023. PMID: 36642087 Free PMC article.
-
Experience with Contraceptive Dosage Forms and Interest in Novel PrEP Technologies in Women.AIDS Behav. 2023 Nov;27(11):3596-3602. doi: 10.1007/s10461-023-04072-6. Epub 2023 May 23. AIDS Behav. 2023. PMID: 37221330 Free PMC article.
References
-
- UNAIDS Confronting inequalities: lessons for pandemic responses from 40 years of AIDS. July 14, 2021. https://www.unaids.org/en/resources/documents/2021/2021-global-aids-upda...
-
- WHO . World Health Organization; Geneva: 2015. Policy brief: pre-exposure prophylaxis (PrEP): WHO expands recommendation on oral pre-exposure prophylaxis of HIV infection (PrEP)
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI154468/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
- UM1 AI069469/AI/NIAID NIH HHS/United States
- UM1 AI069530/AI/NIAID NIH HHS/United States
- UM1 AI069521/AI/NIAID NIH HHS/United States
- UM1 AI068613/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

