Tenascins Interfere With Remyelination in an Ex Vivo Cerebellar Explant Model of Demyelination
- PMID: 35372366
- PMCID: PMC8965512
- DOI: 10.3389/fcell.2022.819967
Tenascins Interfere With Remyelination in an Ex Vivo Cerebellar Explant Model of Demyelination
Abstract
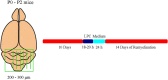
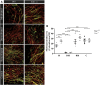
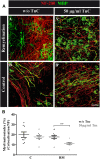
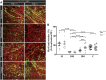
Oligodendrocytes form myelin membranes and thereby secure the insulation of axons and the rapid conduction of action potentials. Diseases such as multiple sclerosis highlight the importance of this glial cell population for brain function. In the adult brain, efficient remyelination following the damage to oligodendrocytes is compromised. Myelination is characterized by proliferation, migration, and proper integration of oligodendrocyte precursor cells (OPCs). These processes are among others controlled by proteins of the extracellular matrix (ECM). As a prominent representative ECM molecule, tenascin-C (Tnc) exerts an inhibitory effect on the migration and differentiation of OPCs. The structurally similar paralogue tenascin-R (Tnr) is known to promote the differentiation of oligodendrocytes. The model of lysolecithin-induced demyelination of cerebellar slice cultures represents an important tool for the analysis of the remyelination process. Ex vivo cerebellar explant cultures of Tnc -/- and Tnr -/- mouse lines displayed enhanced remyelination by forming thicker myelin membranes upon exposure to lysolecithin. The inhibitory effect of tenascins on remyelination could be confirmed when demyelinated wildtype control cultures were exposed to purified Tnc or Tnr protein. In that approach, the remyelination efficiency decreased in a dose-dependent manner with increasing concentrations of ECM molecules added. In order to examine potential roles in a complex in vivo environment, we successfully established cuprizone-based acute demyelination to analyze the remyelination behavior after cuprizone withdrawal in SV129, Tnc -/- , and Tnr -/- mice. In addition, we documented by immunohistochemistry in the cuprizone model the expression of chondroitin sulfate proteoglycans that are inhibitory for the differentiation of OPCs. In conclusion, inhibitory properties of Tnc and Tnr for myelin membrane formation could be demonstrated by using an ex vivo approach.
Keywords: extracellular matrix (ECM); myelin; myelin lesion; oligodendrocyte; regeneration; tenascin-C; tenascin-R.
Copyright © 2022 Bauch, Ort, Ulc and Faissner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model.Cells. 2022 May 28;11(11):1773. doi: 10.3390/cells11111773. Cells. 2022. PMID: 35681468 Free PMC article.
-
Inactivation of Protein Tyrosine Phosphatase Receptor Type Z by Pleiotrophin Promotes Remyelination through Activation of Differentiation of Oligodendrocyte Precursor Cells.J Neurosci. 2015 Sep 2;35(35):12162-71. doi: 10.1523/JNEUROSCI.2127-15.2015. J Neurosci. 2015. PMID: 26338327 Free PMC article.
-
Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination.Ann Neurol. 2012 Sep;72(3):419-32. doi: 10.1002/ana.23599. Ann Neurol. 2012. PMID: 23034914
-
Matrix metalloproteinases shape the oligodendrocyte (niche) during development and upon demyelination.Neurosci Lett. 2020 Jun 11;729:134980. doi: 10.1016/j.neulet.2020.134980. Epub 2020 Apr 19. Neurosci Lett. 2020. PMID: 32315713 Review.
-
Engineering biomaterial microenvironments to promote myelination in the central nervous system.Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
Cited by
-
The guanine nucleotide exchange factor Vav3 intervenes in the migration pathway of oligodendrocyte precursor cells on tenascin-C.Front Cell Dev Biol. 2022 Nov 30;10:1042403. doi: 10.3389/fcell.2022.1042403. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36531963 Free PMC article.
-
IFN-Induced Protein with Tetratricopeptide Repeats 2 Limits Autoimmune Inflammation by Regulating Myeloid Cell Activation and Metabolic Activity.J Immunol. 2023 Mar 15;210(6):721-731. doi: 10.4049/jimmunol.2200746. J Immunol. 2023. PMID: 36695771 Free PMC article.
-
The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model.Cells. 2022 May 28;11(11):1773. doi: 10.3390/cells11111773. Cells. 2022. PMID: 35681468 Free PMC article.
-
The molecular regulation of oligodendrocyte development and CNS myelination by ECM proteins.Front Cell Dev Biol. 2022 Sep 6;10:952135. doi: 10.3389/fcell.2022.952135. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36147746 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

