Expression and Roles of Lynx1, a Modulator of Cholinergic Transmission, in Skeletal Muscles and Neuromuscular Junctions in Mice
- PMID: 35372356
- PMCID: PMC8967655
- DOI: 10.3389/fcell.2022.838612
Expression and Roles of Lynx1, a Modulator of Cholinergic Transmission, in Skeletal Muscles and Neuromuscular Junctions in Mice
Abstract
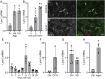
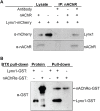
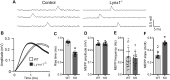
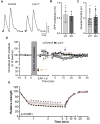
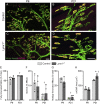
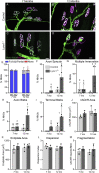
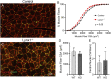
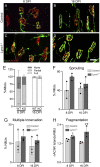
Lynx1 is a glycosylphosphatidylinositol (GPI)-linked protein shown to affect synaptic plasticity through modulation of nicotinic acetylcholine receptor (nAChR) subtypes in the brain. Because of this function and structural similarity to α-bungarotoxin, which binds muscle-specific nAChRs with high affinity, Lynx1 is a promising candidate for modulating nAChRs in skeletal muscles. However, little is known about the expression and roles of Lynx1 in skeletal muscles and neuromuscular junctions (NMJs). Here, we show that Lynx1 is expressed in skeletal muscles, increases during development, and concentrates at NMJs. We also demonstrate that Lynx1 interacts with muscle-specific nAChR subunits. Additionally, we present data indicating that Lynx1 deletion alters the response of skeletal muscles to cholinergic transmission and their contractile properties. Based on these findings, we asked if Lynx1 deletion affects developing and adult NMJs. Loss of Lynx1 had no effect on NMJs at postnatal day 9 (P9) and moderately increased their size at P21. Thus, Lynx1 plays a minor role in the structural development of NMJs. In 7- and 12-month-old mice lacking Lynx1, there is a marked increase in the incidence of NMJs with age- and disease-associated morphological alterations. The loss of Lynx1 also reduced the size of adult muscle fibers. Despite these effects, Lynx1 deletion did not alter the rate of NMJ reinnervation and stability following motor axon injury. These findings suggest that Lynx1 is not required during fast remodeling of the NMJ, as is the case during reformation following crushing of motor axons and development. Instead, these data indicate that the primary role of Lynx1 may be to maintain the structure and function of adult and aging NMJs.
Keywords: Lynx1; acetylcholine receptor; aging; cholinergic transmission; neuromuscular junction; skeletal muscle; synaptic plasticity.
Copyright © 2022 Doss, Barbat-Artigas, Lopes, Pradhan, Prószyński, Robitaille and Valdez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Neuromuscular junctions (NMJs): ultrastructural analysis and nicotinic acetylcholine receptor (nAChR) subunit mRNA expression in offspring subjected to protein restriction throughout pregnancy.Int J Exp Pathol. 2017 Apr;98(2):109-116. doi: 10.1111/iep.12229. Epub 2017 May 25. Int J Exp Pathol. 2017. PMID: 28543723 Free PMC article.
-
Overexpression of Dok-7 in skeletal muscle enhances neuromuscular transmission with structural alterations of neuromuscular junctions: Implications in robustness of neuromuscular transmission.Biochem Biophys Res Commun. 2020 Feb 26;523(1):214-219. doi: 10.1016/j.bbrc.2019.12.011. Epub 2019 Dec 14. Biochem Biophys Res Commun. 2020. PMID: 31848047
-
Muscle Fibers Secrete FGFBP1 to Slow Degeneration of Neuromuscular Synapses during Aging and Progression of ALS.J Neurosci. 2017 Jan 4;37(1):70-82. doi: 10.1523/JNEUROSCI.2992-16.2016. J Neurosci. 2017. PMID: 28053031 Free PMC article.
-
Assembly, plasticity and selective vulnerability to disease of mouse neuromuscular junctions.J Neurocytol. 2003 Jun-Sep;32(5-8):849-62. doi: 10.1023/B:NEUR.0000020628.36013.88. J Neurocytol. 2003. PMID: 15034272 Review.
-
Lynx1 prototoxins: critical accessory proteins of neuronal nicotinic acetylcholine receptors.Curr Opin Pharmacol. 2021 Feb;56:46-51. doi: 10.1016/j.coph.2020.09.016. Epub 2020 Nov 27. Curr Opin Pharmacol. 2021. PMID: 33254061 Free PMC article. Review.
Cited by
-
Comparison of Conformations and Interactions with Nicotinic Acetylcholine Receptors for E. coli-Produced and Synthetic Three-Finger Protein SLURP-1.Int J Mol Sci. 2023 Nov 29;24(23):16950. doi: 10.3390/ijms242316950. Int J Mol Sci. 2023. PMID: 38069271 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

