The Actin Regulators Involved in the Function and Related Diseases of Lymphocytes
- PMID: 35371070
- PMCID: PMC8965893
- DOI: 10.3389/fimmu.2022.799309
The Actin Regulators Involved in the Function and Related Diseases of Lymphocytes
Abstract
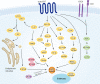
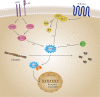
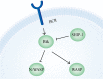
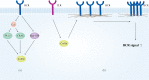
Actin is an important cytoskeletal protein involved in signal transduction, cell structure and motility. Actin regulators include actin-monomer-binding proteins, Wiskott-Aldrich syndrome (WAS) family of proteins, nucleation proteins, actin filament polymerases and severing proteins. This group of proteins regulate the dynamic changes in actin assembly/disassembly, thus playing an important role in cell motility, intracellular transport, cell division and other basic cellular activities. Lymphocytes are important components of the human immune system, consisting of T-lymphocytes (T cells), B-lymphocytes (B cells) and natural killer cells (NK cells). Lymphocytes are indispensable for both innate and adaptive immunity and cannot function normally without various actin regulators. In this review, we first briefly introduce the structure and fundamental functions of a variety of well-known and newly discovered actin regulators, then we highlight the role of actin regulators in T cell, B cell and NK cell, and finally provide a landscape of various diseases associated with them. This review provides new directions in exploring actin regulators and promotes more precise and effective treatments for related diseases.
Keywords: B cell; NK cell; T cell; WAS; actin regulators.
Copyright © 2022 Sun, Zhong, Fu, Miller, Lee, Yu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Biochemical Activities of the Wiskott-Aldrich Syndrome Homology Region 2 Domains of Sarcomere Length Short (SALS) Protein.J Biol Chem. 2016 Jan 8;291(2):667-80. doi: 10.1074/jbc.M115.683904. Epub 2015 Nov 17. J Biol Chem. 2016. PMID: 26578512 Free PMC article.
-
The Wiskott-Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF-kappa B (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement.J Immunol. 2005 Mar 1;174(5):2602-11. doi: 10.4049/jimmunol.174.5.2602. J Immunol. 2005. PMID: 15728466
-
A peptide derived from the Wiskott-Aldrich syndrome (WAS) protein-interacting protein (WIP) restores WAS protein level and actin cytoskeleton reorganization in lymphocytes from patients with WAS mutations that disrupt WIP binding.J Allergy Clin Immunol. 2011 Apr;127(4):998-1005.e1-2. doi: 10.1016/j.jaci.2011.01.015. Epub 2011 Mar 3. J Allergy Clin Immunol. 2011. PMID: 21376381 Free PMC article.
-
Wiskott-Aldrich syndrome protein--dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes.Immunol Rev. 2013 Nov;256(1):10-29. doi: 10.1111/imr.12112. Immunol Rev. 2013. PMID: 24117810 Review.
-
Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins.Annu Rev Biochem. 2001;70:649-76. doi: 10.1146/annurev.biochem.70.1.649. Annu Rev Biochem. 2001. PMID: 11395419 Review.
Cited by
-
Proteome identification of common immunological proteins of two nematode parasites.Parasites Hosts Dis. 2024 Aug;62(3):342-350. doi: 10.3347/PHD.24027. Epub 2024 Aug 26. Parasites Hosts Dis. 2024. PMID: 39218633 Free PMC article.
-
Expression of non-phosphorylatable S5A-L-plastin exerts phenotypes distinct from L-plastin deficiency during podosome formation and phagocytosis.Front Cell Dev Biol. 2023 Apr 17;11:1020091. doi: 10.3389/fcell.2023.1020091. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37138794 Free PMC article.
-
Alterations of lipid-related genes during anti-tuberculosis treatment: insights into host immune responses and potential transcriptional biomarkers.Front Immunol. 2023 Oct 31;14:1210372. doi: 10.3389/fimmu.2023.1210372. eCollection 2023. Front Immunol. 2023. PMID: 38022579 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

