Anti-FcαRI Monoclonal Antibodies Resolve IgA Autoantibody-Mediated Disease
- PMID: 35371001
- PMCID: PMC8965572
- DOI: 10.3389/fimmu.2022.732977
Anti-FcαRI Monoclonal Antibodies Resolve IgA Autoantibody-Mediated Disease
Abstract
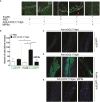
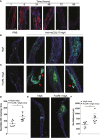
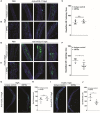
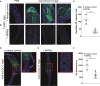
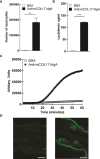
Immunoglobulin A (IgA) is generally considered as a non-inflammatory regulator of mucosal immunity, and its importance in diversifying the gut microbiota is increasingly appreciated. IgA autoantibodies have been found in several autoimmune or chronic inflammatory diseases, but their role in pathophysiology is ill-understood. IgA can interact with the Fc receptor FcαRI on immune cells. We now established a novel IgA autoimmune blistering model, which closely resembles the human disease linear IgA bullous disease (LABD) by using genetically modified mice that produce human IgA and express human FcαRI. Intravital microscopy demonstrated that presence of IgA anti-collagen XVII, - the auto-antigen in LABD-, resulted in neutrophil activation and extravasation from blood vessels into skin tissue. Continued exposure to anti-collagen XVII IgA led to massive neutrophil accumulation, severe tissue damage and blister formation. Importantly, treatment with anti-FcαRI monoclonal antibodies not only prevented disease, but was also able to resolve existing inflammation and tissue damage. Collectively, our data reveal a novel role of neutrophil FcαRI in IgA autoantibody-mediated disease and identify FcαRI as promising new therapeutic target to resolve chronic inflammation and tissue damage.
Keywords: CD89; FcαRI; IgA; LABD; neutrophils.
Copyright © 2022 Bos, Aleyd, van der Steen, Winter, Heemskerk, Pouw, Boon, Musters, Bakema, Sitaru, Cogné and van Egmond.
Conflict of interest statement
Author LB was employed by company JJP Biologics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Antagonizing FcαR1 (CD89) as treatment in IgA-mediated chronic inflammation and autoimmunity.Front Immunol. 2023 Apr 4;14:1118539. doi: 10.3389/fimmu.2023.1118539. eCollection 2023. Front Immunol. 2023. PMID: 37081893 Free PMC article.
-
Peptide mimetics of immunoglobulin A (IgA) and FcαRI block IgA-induced human neutrophil activation and migration.Eur J Immunol. 2017 Oct;47(10):1835-1845. doi: 10.1002/eji.201646782. Epub 2017 Sep 6. Eur J Immunol. 2017. PMID: 28736835 Free PMC article.
-
IgA and FcαRI: Pathological Roles and Therapeutic Opportunities.Front Immunol. 2019 Mar 22;10:553. doi: 10.3389/fimmu.2019.00553. eCollection 2019. Front Immunol. 2019. PMID: 30984170 Free PMC article. Review.
-
The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease.Immunol Rev. 2015 Nov;268(1):123-38. doi: 10.1111/imr.12337. Immunol Rev. 2015. PMID: 26497517 Review.
-
Immunoglobulin A: magic bullet or Trojan horse?Eur J Clin Invest. 2017 Feb;47(2):184-192. doi: 10.1111/eci.12716. Epub 2017 Jan 19. Eur J Clin Invest. 2017. PMID: 28024097 Review.
Cited by
-
Fcα Receptor-1-Activated Monocytes Promote B Lymphocyte Migration and IgA Isotype Switching.Int J Mol Sci. 2022 Sep 22;23(19):11132. doi: 10.3390/ijms231911132. Int J Mol Sci. 2022. PMID: 36232432 Free PMC article.
-
Antagonizing FcαR1 (CD89) as treatment in IgA-mediated chronic inflammation and autoimmunity.Front Immunol. 2023 Apr 4;14:1118539. doi: 10.3389/fimmu.2023.1118539. eCollection 2023. Front Immunol. 2023. PMID: 37081893 Free PMC article.
-
Borrelia-specific antibody profiles and complement deposition in joint fluid distinguish antibiotic-refractory from -responsive Lyme arthritis.iScience. 2024 Jan 4;27(2):108804. doi: 10.1016/j.isci.2024.108804. eCollection 2024 Feb 16. iScience. 2024. PMID: 38303696 Free PMC article.
-
IgA-producing B cells in lung homeostasis and disease.Front Immunol. 2023 Mar 1;14:1117749. doi: 10.3389/fimmu.2023.1117749. eCollection 2023. Front Immunol. 2023. PMID: 36936934 Free PMC article. Review.
-
Anti-NC16A IgA from Patients with Linear IgA Bullous Dermatosis Induce Neutrophil-Dependent Subepidermal Blistering in Mice.J Invest Dermatol. 2024 Jan;144(1):24-32.e1. doi: 10.1016/j.jid.2023.05.027. Epub 2023 Jul 10. J Invest Dermatol. 2024. PMID: 37437774 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

