Epidermal Growth Factor Pathway in the Age-Related Decline of Oligodendrocyte Regeneration
- PMID: 35370556
- PMCID: PMC8968959
- DOI: 10.3389/fncel.2022.838007
Epidermal Growth Factor Pathway in the Age-Related Decline of Oligodendrocyte Regeneration
Abstract
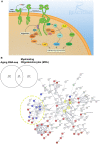
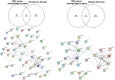
Oligodendrocytes (OLs) are specialized glial cells that myelinate CNS axons. OLs are generated throughout life from oligodendrocyte progenitor cells (OPCs) via a series of tightly controlled differentiation steps. Life-long myelination is essential for learning and to replace myelin lost in age-related pathologies such as Alzheimer's disease (AD) as well as white matter pathologies such as multiple sclerosis (MS). Notably, there is considerable myelin loss in the aging brain, which is accelerated in AD and underpins the failure of remyelination in secondary progressive MS. An important factor in age-related myelin loss is a marked decrease in the regenerative capacity of OPCs. In this review, we will contextualize recent advances in the key role of Epidermal Growth Factor (EGF) signaling in regulating multiple biological pathways in oligodendroglia that are dysregulated in aging.
Keywords: EGF; EGFR; ErbB; aging; myelin; oligodendrocyte; white matter.
Copyright © 2022 Rivera, Azim, Macchi, Porzionato, Butt and De Caro.
Conflict of interest statement
AR and AB are shareholders of Gliagenesis LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain.Cells. 2022 May 31;11(11):1809. doi: 10.3390/cells11111809. Cells. 2022. PMID: 35681504 Free PMC article. Review.
-
Keeping the ageing brain wired: a role for purine signalling in regulating cellular metabolism in oligodendrocyte progenitors.Pflugers Arch. 2021 May;473(5):775-783. doi: 10.1007/s00424-021-02544-z. Epub 2021 Mar 13. Pflugers Arch. 2021. PMID: 33712969 Free PMC article. Review.
-
Loss of Tuberous Sclerosis Complex1 in Adult Oligodendrocyte Progenitor Cells Enhances Axon Remyelination and Increases Myelin Thickness after a Focal Demyelination.J Neurosci. 2017 Aug 2;37(31):7534-7546. doi: 10.1523/JNEUROSCI.3454-16.2017. Epub 2017 Jul 10. J Neurosci. 2017. PMID: 28694334 Free PMC article.
-
Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain.Geroscience. 2023 Feb;45(1):249-264. doi: 10.1007/s11357-022-00621-4. Epub 2022 Aug 5. Geroscience. 2023. PMID: 35930094 Free PMC article.
-
Oligodendrocyte Development and Regenerative Therapeutics in Multiple Sclerosis.Life (Basel). 2021 Apr 9;11(4):327. doi: 10.3390/life11040327. Life (Basel). 2021. PMID: 33918664 Free PMC article. Review.
Cited by
-
Navigating the metabolic maze: anomalies in fatty acid and cholesterol processes in Alzheimer's astrocytes.Alzheimers Res Ther. 2024 Mar 23;16(1):63. doi: 10.1186/s13195-024-01430-x. Alzheimers Res Ther. 2024. PMID: 38521950 Free PMC article. Review.
-
The Genomic Intersection of Oligodendrocyte Dynamics in Schizophrenia and Aging Unravels Novel Pathological Mechanisms and Therapeutic Potentials.Int J Mol Sci. 2024 Apr 18;25(8):4452. doi: 10.3390/ijms25084452. Int J Mol Sci. 2024. PMID: 38674040 Free PMC article. Review.
-
Proteomics analysis of periplaque and chronic inactive multiple sclerosis lesions.Front Mol Neurosci. 2024 Aug 21;17:1448215. doi: 10.3389/fnmol.2024.1448215. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39234409 Free PMC article.
-
Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain.Cells. 2022 May 31;11(11):1809. doi: 10.3390/cells11111809. Cells. 2022. PMID: 35681504 Free PMC article. Review.
-
Genome-Wide Mapping Implicates 5-Hydroxymethylcytosines in Diabetes Mellitus and Alzheimer's Disease.J Alzheimers Dis. 2023;93(3):1135-1151. doi: 10.3233/JAD-221113. J Alzheimers Dis. 2023. PMID: 37182870 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

