Dimers of isatin derived α-methylene-γ-butyrolactone as potent anti-cancer agents
- PMID: 35367592
- PMCID: PMC9037195
- DOI: 10.1016/j.bmcl.2022.128713
Dimers of isatin derived α-methylene-γ-butyrolactone as potent anti-cancer agents
Abstract
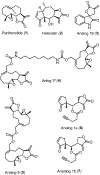
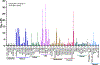
The IKK-NFκB complex is a key signaling node that facilitates activation of gene expression in response to extracellular signals. The kinase IKKβ and the transcription factor RELA have been targeted by covalent modifiers that bind to surface exposed cysteine residues. A common feature in well characterized covalent modifiers of RELA and IKKβ is the Michael acceptor containing α-methylene-γ-butyrolactone functionality. Through synthesis and evaluation of a focused set of α-methylene-γ-butyrolactone containing spirocyclic dimers (SpiDs) we identified SpiD3 as an anticancer agent with low nanomolar potency. Using cell-free and cell-based studies we show that SpiD3 is a covalent modifier that generates stable RELA containing high molecular weight complexes. SpiD3 inhibits TNFα-induced IκBα phosphorylation resulting in the blockade of RELA nuclear translocation. SpiD3 induces apoptosis, inhibits colony formation and migration of cancer cells. The NCI-60 cell line screen revealed that SpiD3 potently inhibits growth of leukemia cell lines, making it a suitable pre-therapeutic lead for hematological malignancies.
Keywords: Cancer; Irreversible inhibitor; NFκB pathway; RELA inhibitor; Spirocyclic compounds; α-methylene-γ-butyrolactone.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures


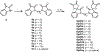
Similar articles
-
Isatin Derived Spirocyclic Analogues with α-Methylene-γ-butyrolactone as Anticancer Agents: A Structure-Activity Relationship Study.J Med Chem. 2016 May 26;59(10):5121-7. doi: 10.1021/acs.jmedchem.6b00400. Epub 2016 Apr 26. J Med Chem. 2016. PMID: 27077228 Free PMC article.
-
Inhibition of ICAM-1 gene expression, monocyte adhesion and cancer cell invasion by targeting IKK complex: molecular and functional study of novel alpha-methylene-gamma-butyrolactone derivatives.Carcinogenesis. 2004 Oct;25(10):1925-34. doi: 10.1093/carcin/bgh211. Epub 2004 Jun 24. Carcinogenesis. 2004. PMID: 15217903
-
Trichothecin induces cell death in NF-κB constitutively activated human cancer cells via inhibition of IKKβ phosphorylation.PLoS One. 2013 Aug 1;8(8):e71333. doi: 10.1371/journal.pone.0071333. Print 2013. PLoS One. 2013. PMID: 23936501 Free PMC article.
-
Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activation.Biochem Pharmacol. 2005 Nov 15;70(10):1443-57. doi: 10.1016/j.bcp.2005.08.011. Epub 2005 Sep 21. Biochem Pharmacol. 2005. PMID: 16181613
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
Design, synthesis, and evaluation of a mitoxantrone probe (MXP) for biological studies.Bioorg Med Chem Lett. 2023 Oct 1;94:129465. doi: 10.1016/j.bmcl.2023.129465. Epub 2023 Sep 3. Bioorg Med Chem Lett. 2023. PMID: 37669721 Free PMC article.
-
The Novel Anti-Cancer Agent, SpiD3, Is Cytotoxic in CLL Cells Resistant to Ibrutinib or Venetoclax.Hemato. 2024 Sep;5(3):321-339. doi: 10.3390/hemato5030024. Epub 2024 Aug 27. Hemato. 2024. PMID: 39450301 Free PMC article.
-
Domino Reactions Enable Zn-Mediated Direct Synthesis of Spiro-Fused 2-Oxindole-α-Methylene-γ-Butyrolactones/Lactams from Isatin Derivatives and 2-(Bromomethyl)acrylates.Molecules. 2024 Jul 30;29(15):3612. doi: 10.3390/molecules29153612. Molecules. 2024. PMID: 39125017 Free PMC article.
-
Mycobacterium tuberculosis CitA activity is modulated by cysteine oxidation and pyruvate binding.RSC Med Chem. 2023 Apr 5;14(5):921-933. doi: 10.1039/d3md00058c. eCollection 2023 May 25. RSC Med Chem. 2023. PMID: 37252106 Free PMC article.
References
-
- DiDonato JA; Hayakawa M; Rothwarf DM; Zandi E; Karin M A cytokine-responsive IkB kinase that activates the transcription factor NF-kB. Nature 1997, 388, 549–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

