Virus-Specific Regulatory T Cells Persist as Memory in a Neurotropic Coronavirus Infection
- PMID: 35365567
- PMCID: PMC9012697
- DOI: 10.4049/jimmunol.2100794
Virus-Specific Regulatory T Cells Persist as Memory in a Neurotropic Coronavirus Infection
Abstract
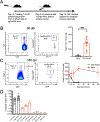
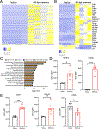
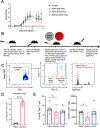
Regulatory T cells (Tregs) are critical for regulating immunopathogenic responses in a variety of infections, including infection of mice with JHM strain of mouse hepatitis virus (JHMV), a neurotropic coronavirus that causes immune-mediated demyelinating disease. Although virus-specific Tregs are known to mitigate disease in this infection by suppressing pathogenic effector T cell responses of the same specificity, it is unclear whether these virus-specific Tregs form memory populations and persist similar to their conventional T cell counterparts of the same epitope specificity. Using congenically labeled JHMV-specific Tregs, we found that virus-specific Tregs persist long-term after murine infection, through at least 180 d postinfection and stably maintain Foxp3 expression. We additionally demonstrate that these cells are better able to proliferate and inhibit virus-specific T cell responses postinfection than naive Tregs of the same specificity, further suggesting that these cells differentiate into memory Tregs upon encountering cognate Ag. Taken together, these data suggest that virus-specific Tregs are able to persist long-term in the absence of viral Ag as memory Tregs.
Copyright © 2022 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures

Similar articles
-
Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus.J Immunol. 2010 Apr 15;184(8):4391-400. doi: 10.4049/jimmunol.0903918. Epub 2010 Mar 5. J Immunol. 2010. PMID: 20208000 Free PMC article.
-
Characterization of mouse hepatitis virus-specific cytotoxic T cells derived from the central nervous system of mice infected with the JHM strain.J Virol. 1993 Dec;67(12):7050-9. doi: 10.1128/JVI.67.12.7050-7059.1993. J Virol. 1993. PMID: 8230429 Free PMC article.
-
The art of survival during viral persistence.J Neurovirol. 2002 Dec;8 Suppl 2:53-8. doi: 10.1080/13550280290167884. J Neurovirol. 2002. PMID: 12491152
-
Role of cytotoxic T lymphocytes and interferon-γ in coronavirus infection: Lessons from murine coronavirus infections in mice.J Vet Med Sci. 2020 Oct 20;82(10):1410-1414. doi: 10.1292/jvms.20-0313. Epub 2020 Aug 5. J Vet Med Sci. 2020. PMID: 32759577 Free PMC article. Review.
-
MHV infection of the CNS: mechanisms of immune-mediated control.Viral Immunol. 2001;14(1):1-18. doi: 10.1089/08828240151061329. Viral Immunol. 2001. PMID: 11270593 Review.
Cited by
-
Keeping T cell memories in mind.Trends Immunol. 2022 Dec;43(12):1018-1031. doi: 10.1016/j.it.2022.10.001. Epub 2022 Nov 8. Trends Immunol. 2022. PMID: 36369103 Free PMC article. Review.
References
-
- Savage PA, Klawon DEJ, and Miller CH. 2020. Regulatory T Cell Development. Annu. Rev. Immunol 38: 421–453. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

