A secreted ribonuclease effector from Verticillium dahliae localizes in the plant nucleus to modulate host immunity
- PMID: 35363930
- PMCID: PMC9276946
- DOI: 10.1111/mpp.13213
A secreted ribonuclease effector from Verticillium dahliae localizes in the plant nucleus to modulate host immunity
Abstract
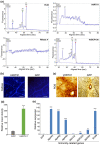
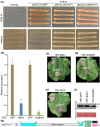
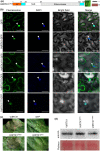
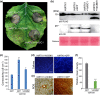
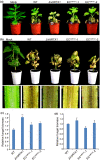
The arms race between fungal pathogens and plant hosts involves recognition of fungal effectors to induce host immunity. Although various fungal effectors have been identified, the effector functions of ribonucleases are largely unknown. Herein, we identified a ribonuclease secreted by Verticillium dahliae (VdRTX1) that translocates into the plant nucleus to modulate immunity. The activity of VdRTX1 causes hypersensitive response (HR)-related cell death in Nicotiana benthamiana and cotton. VdRTX1 possesses a signal peptide but is unlikely to be an apoplastic effector because its nuclear localization in the plant is necessary for cell death induction. Knockout of VdRTX1 significantly enhanced V. dahliae virulence on tobacco while V. dahliae employs the known suppressor VdCBM1 to escape the immunity induced by VdRTX1. VdRTX1 homologs are widely distributed in fungi but transient expression of 24 homologs from other fungi did not yield cell death induction, suggesting that this function is specific to the VdRTX1 in V. dahliae. Expression of site-directed mutants of VdRTX1 in N. benthamiana leaves revealed conserved ligand-binding sites that are important for VdRTX1 function in inducing cell death. Thus, VdRTX1 functions as a unique HR-inducing effector in V. dahliae that contributes to the activation of plant immunity.
Keywords: Verticillium dahliae; VdRTX1; host immunity; secreted ribonuclease.
© 2022 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
The Verticillium-specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections.New Phytol. 2017 Jul;215(1):368-381. doi: 10.1111/nph.14537. Epub 2017 Apr 13. New Phytol. 2017. PMID: 28407259
-
Verticillium dahliae Effector VdCE11 Contributes to Virulence by Promoting Accumulation and Activity of the Aspartic Protease GhAP1 from Cotton.Microbiol Spectr. 2023 Feb 14;11(1):e0354722. doi: 10.1128/spectrum.03547-22. Epub 2023 Jan 19. Microbiol Spectr. 2023. PMID: 36656049 Free PMC article.
-
Verticillium dahliae secreted protein Vd424Y is required for full virulence, targets the nucleus of plant cells, and induces cell death.Mol Plant Pathol. 2021 Sep;22(9):1109-1120. doi: 10.1111/mpp.13100. Epub 2021 Jul 7. Mol Plant Pathol. 2021. PMID: 34233072 Free PMC article.
-
The secretome of Verticillium dahliae in collusion with plant defence responses modulates Verticillium wilt symptoms.Biol Rev Camb Philos Soc. 2022 Oct;97(5):1810-1822. doi: 10.1111/brv.12863. Epub 2022 Apr 27. Biol Rev Camb Philos Soc. 2022. PMID: 35478378 Free PMC article. Review.
-
An Overview of the Molecular Genetics of Plant Resistance to the Verticillium Wilt Pathogen Verticillium dahliae.Int J Mol Sci. 2020 Feb 7;21(3):1120. doi: 10.3390/ijms21031120. Int J Mol Sci. 2020. PMID: 32046212 Free PMC article. Review.
Cited by
-
Colletotrichum fructicola co-opts cytotoxic ribonucleases that antagonize host competitive microorganisms to promote infection.mBio. 2024 Aug 14;15(8):e0105324. doi: 10.1128/mbio.01053-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953357 Free PMC article.
-
The Secreted Ribonuclease SRE1 Contributes to Setosphaeria turcica Virulence and Activates Plant Immunity.Front Microbiol. 2022 Jul 8;13:941991. doi: 10.3389/fmicb.2022.941991. eCollection 2022. Front Microbiol. 2022. PMID: 35875548 Free PMC article.
-
Nuclear Effectors in Plant Pathogenic Fungi.Mycobiology. 2022 Sep 20;50(5):259-268. doi: 10.1080/12298093.2022.2118928. eCollection 2022. Mycobiology. 2022. PMID: 36404902 Free PMC article. Review.
-
Small secreted effector protein from Fusarium sacchari suppresses host immune response by inhibiting ScPi21-induced cell death.Mol Plant Pathol. 2024 Jan;25(1):e13414. doi: 10.1111/mpp.13414. Mol Plant Pathol. 2024. PMID: 38279852 Free PMC article.
-
Two Metalloproteases VdM35-1 and VdASPF2 from Verticillium dahliae Are Required for Fungal Pathogenicity, Stress Adaptation, and Activating Immune Response of Host.Microbiol Spectr. 2022 Dec 21;10(6):e0247722. doi: 10.1128/spectrum.02477-22. Epub 2022 Oct 12. Microbiol Spectr. 2022. PMID: 36222688 Free PMC article.
References
-
- Albert, I. , Böhm, H. , Albert, M. , Feiler, C.E. , Imkampe, J. , Wallmeroth, N. et al. (2015) An RLP23‐SOBIR1‐BAK1 complex mediates NLP‐triggered immunity. Nature Plants, 1, 15140. - PubMed
-
- Białas, A. , Zess, E.K. , De la Concepcion, J.C. , Franceschetti, M. , Pennington, H.G. , Yoshida, K. et al. (2018) Lessons in effector and NLR biology of plant–microbe systems. Molecular Plant‐Microbe Interactions, 31, 34–45. - PubMed
-
- Boller, T. & Felix, G. (2009) A renaissance of elicitors. Perception of microbe‐associated molecular patterns and danger signals by pattern recognition receptors. Annual Review of Plant Biology, 60, 379–406. - PubMed
-
- Brandhorst, T.T. & Kenealy, W.R. (1992) Production and localization of restrictocinin Aspergillus restrictus . Journal of General Microbiology, 138, 1429–1435. - PubMed
-
- Cao, Y.‐F. , Zhu, X.‐W. , Xiang, Y.U. , Li, D.‐Q. , Yang, J.‐R. , Mao, Q.‐Z. et al. (2011) Genomic characterization of a novel dsRNA virus detected in the phytopathogenic fungus Verticillium dahliae Kleb. Virus Research, 159, 73–78. - PubMed

