The Impact of the Secondary Binding Pocket on the Pharmacology of Class A GPCRs
- PMID: 35355719
- PMCID: PMC8959758
- DOI: 10.3389/fphar.2022.847788
The Impact of the Secondary Binding Pocket on the Pharmacology of Class A GPCRs
Abstract
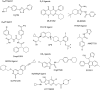
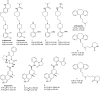
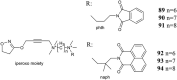
G-protein coupled receptors (GPCRs) are considered important therapeutic targets due to their pathophysiological significance and pharmacological relevance. Class A receptors represent the largest group of GPCRs that gives the highest number of validated drug targets. Endogenous ligands bind to the orthosteric binding pocket (OBP) embedded in the intrahelical space of the receptor. During the last 10 years, however, it has been turned out that in many receptors there is secondary binding pocket (SBP) located in the extracellular vestibule that is much less conserved. In some cases, it serves as a stable allosteric site harbouring allosteric ligands that modulate the pharmacology of orthosteric binders. In other cases it is used by bitopic compounds occupying both the OBP and SBP. In these terms, SBP binding moieties might influence the pharmacology of the bitopic ligands. Together with others, our research group showed that SBP binders contribute significantly to the affinity, selectivity, functional activity, functional selectivity and binding kinetics of bitopic ligands. Based on these observations we developed a structure-based protocol for designing bitopic compounds with desired pharmacological profile.
Keywords: GPCR (G-protein coupled receptor); allosteric; bitopic; functional selectivity; selectivity.
Copyright © 2022 Egyed, Kiss and Keserű.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
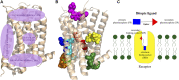
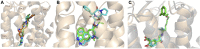

Similar articles
-
Controlling the selectivity of aminergic GPCR ligands from the extracellular vestibule.Bioorg Chem. 2021 Jun;111:104832. doi: 10.1016/j.bioorg.2021.104832. Epub 2021 Mar 19. Bioorg Chem. 2021. PMID: 33826962
-
Recent Advances in the Drug Discovery and Development of Dualsteric/ Bitopic Activators of G Protein-Coupled Receptors.Curr Top Med Chem. 2019;19(26):2378-2392. doi: 10.2174/1568026619666191009164609. Curr Top Med Chem. 2019. PMID: 31833462 Review.
-
Molecular alliance-from orthosteric and allosteric ligands to dualsteric/bitopic agonists at G protein coupled receptors.Angew Chem Int Ed Engl. 2013 Jan 7;52(2):508-16. doi: 10.1002/anie.201205315. Epub 2012 Dec 6. Angew Chem Int Ed Engl. 2013. PMID: 23225228 Review.
-
Orthosteric/allosteric bitopic ligands: going hybrid at GPCRs.Mol Interv. 2009 Jun;9(3):125-35. doi: 10.1124/mi.9.3.6. Mol Interv. 2009. PMID: 19592673 Review.
-
Bridging the gap: bitopic ligands of G-protein-coupled receptors.Trends Pharmacol Sci. 2013 Jan;34(1):59-66. doi: 10.1016/j.tips.2012.10.003. Epub 2012 Nov 20. Trends Pharmacol Sci. 2013. PMID: 23177916 Review.
Cited by
-
Allosteric Regulation of G-Protein-Coupled Receptors: From Diversity of Molecular Mechanisms to Multiple Allosteric Sites and Their Ligands.Int J Mol Sci. 2023 Mar 24;24(7):6187. doi: 10.3390/ijms24076187. Int J Mol Sci. 2023. PMID: 37047169 Free PMC article. Review.
-
Linkers in Bitopic Agonists Shape Bias Profile among Transducers for the Dopamine D2 and D3 Receptors.ACS Pharmacol Transl Sci. 2024 Jul 26;7(8):2333-2349. doi: 10.1021/acsptsci.4c00119. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144557
-
Structural Insights into the Unexpected Agonism of Tetracyclic Antidepressants at Serotonin Receptors 5-HT1eR and 5-HT1FR.bioRxiv [Preprint]. 2023 Oct 7:2023.10.05.561100. doi: 10.1101/2023.10.05.561100. bioRxiv. 2023. Update in: Sci Adv. 2024 Apr 19;10(16):eadk4855. doi: 10.1126/sciadv.adk4855 PMID: 37986777 Free PMC article. Updated. Preprint.
-
GPCR activation and GRK2 assembly by a biased intracellular agonist.Nature. 2023 Aug;620(7974):676-681. doi: 10.1038/s41586-023-06395-9. Epub 2023 Aug 2. Nature. 2023. PMID: 37532940
-
Structural insights into the unexpected agonism of tetracyclic antidepressants at serotonin receptors 5-HT1eR and 5-HT1FR.Sci Adv. 2024 Apr 19;10(16):eadk4855. doi: 10.1126/sciadv.adk4855. Epub 2024 Apr 17. Sci Adv. 2024. PMID: 38630816 Free PMC article.
References
-
- Ackley M. A. (2008). Morpholine Dopamine Agonists for the Treatment of Pain. WO 087512 A1.
-
- Allerton C. M. N., Cook A. S., Hepworth D., Miller D. C. (2005). Aminopyridine Derivatives as Selective Dopamine D3 Agonists. WO 115985 Al.
-
- Barnes N. M., Ahern G. P., Becamel C., Bockaert J., Camilleri M., Chaumont-Dubel S., et al. (2021). International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-Hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 73 (1), 310–520. 10.1124/pr.118.015552 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

