Novel Nongenetic Murine Model of Hyperglycemia and Hyperlipidemia-Associated Aggravated Atherosclerosis
- PMID: 35350534
- PMCID: PMC8957812
- DOI: 10.3389/fcvm.2022.813215
Novel Nongenetic Murine Model of Hyperglycemia and Hyperlipidemia-Associated Aggravated Atherosclerosis
Abstract
Objective: Atherosclerosis, the main pathology underlying cardiovascular diseases is accelerated in diabetic patients. Genetic mouse models require breeding efforts which are time-consuming and costly. Our aim was to establish a new nongenetic model of inducible metabolic risk factors that mimics hyperlipidemia, hyperglycemia, or both and allows the detection of phenotypic differences dependent on the metabolic stressor(s).
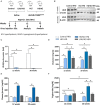
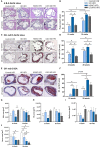
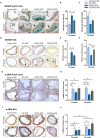
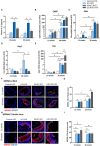
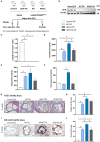
Methods and results: Wild-type mice were injected with gain-of-function PCSK9D377Y (proprotein convertase subtilisin/kexin type 9) mutant adeno-associated viral particles (AAV) and streptozotocin and fed either a high-fat diet (HFD) for 12 or 20 weeks or a high-cholesterol/high-fat diet (Paigen diet, PD) for 8 weeks. To evaluate atherosclerosis, two different vascular sites (aortic sinus and the truncus of the brachiocephalic artery) were examined in the mice. Combined hyperlipidemic and hyperglycemic (HGHCi) mice fed a HFD or PD displayed characteristic features of aggravated atherosclerosis when compared to hyperlipidemia (HCi HFD or PD) mice alone. Atherosclerotic plaques of HGHCi HFD animals were larger, showed a less stable phenotype (measured by the increased necrotic core area, reduced fibrous cap thickness, and less α-SMA-positive area) and had more inflammation (increased plasma IL-1β level, aortic pro-inflammatory gene expression, and MOMA-2-positive cells in the BCA) after 20 weeks of HFD. Differences between the HGHCi and HCi HFD models were confirmed using RNA-seq analysis of aortic tissue, revealing that significantly more genes were dysregulated in mice with combined hyperlipidemia and hyperglycemia than in the hyperlipidemia-only group. The HGHCi-associated genes were related to pathways regulating inflammation (increased Cd68, iNos, and Tnfa expression) and extracellular matrix degradation (Adamts4 and Mmp14). When comparing HFD with PD, the PD aggravated atherosclerosis to a greater extent in mice and showed plaque formation after 8 weeks. Hyperlipidemic and hyperglycemic mice fed a PD (HGHCi PD) showed less collagen (Sirius red) and increased inflammation (CD68-positive cells) within aortic plaques than hyperlipidemic mice (HCi PD). HGHCi-PD mice represent a directly inducible hyperglycemic atherosclerosis model compared with HFD-fed mice, in which atherosclerosis is severe by 8 weeks.
Conclusion: We established a nongenetically inducible mouse model allowing comparative analyses of atherosclerosis in HCi and HGHCi conditions and its modification by diet, allowing analyses of multiple metabolic hits in mice.
Keywords: PCSK9; animal model of disease; atherosclerosis; diabetes; hyperglycemia; hyperlipidemia; streptozotocin.
Copyright © 2022 Gaul, Shahzad, Medert, Gadi, Mäder, Schumacher, Wirth, Ambreen, Fatima, Boeckel, Khawaja, Haas, Brune, Nawroth, Isermann, Laufs and Freichel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):50-9. doi: 10.1161/ATVBAHA.114.303617. Epub 2014 Oct 23. Arterioscler Thromb Vasc Biol. 2015. PMID: 25341796
-
A novel mouse model of diabetes, atherosclerosis and fatty liver disease using an AAV8-PCSK9-D377Y injection and dietary manipulation in db/db mice.Biochem Biophys Res Commun. 2022 Sep 24;622:163-169. doi: 10.1016/j.bbrc.2022.07.031. Epub 2022 Jul 15. Biochem Biophys Res Commun. 2022. PMID: 35868060
-
ZeXieYin formula alleviates atherosclerosis by inhibiting the MAPK/NF-κB signaling pathway in APOE-/- mice to attenuate vascular inflammation and increase plaque stability.J Ethnopharmacol. 2024 Jun 12;327:117969. doi: 10.1016/j.jep.2024.117969. Epub 2024 Mar 2. J Ethnopharmacol. 2024. PMID: 38437888
-
New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway.Atherosclerosis. 2017 Jul;262:113-122. doi: 10.1016/j.atherosclerosis.2017.04.023. Epub 2017 Apr 29. Atherosclerosis. 2017. PMID: 28535426
-
The AAV-PCSK9 murine model of atherosclerosis and metabolic dysfunction.Eur Heart J Open. 2022 Apr 20;2(3):oeac028. doi: 10.1093/ehjopen/oeac028. eCollection 2022 May. Eur Heart J Open. 2022. PMID: 35919346 Free PMC article.
Cited by
-
Interventional hepatic apoC-III knockdown improves atherosclerotic plaque stability and remodeling by triglyceride lowering.JCI Insight. 2022 Jul 8;7(13):e158414. doi: 10.1172/jci.insight.158414. JCI Insight. 2022. PMID: 35653195 Free PMC article.
-
Hypercoagulability Impairs Plaque Stability in Diabetes-Induced Atherosclerosis.Nutrients. 2022 May 10;14(10):1991. doi: 10.3390/nu14101991. Nutrients. 2022. PMID: 35631132 Free PMC article.
-
Membrane-bound Interleukin-1α mediates leukocyte adhesion during atherogenesis.Front Immunol. 2023 Aug 28;14:1252384. doi: 10.3389/fimmu.2023.1252384. eCollection 2023. Front Immunol. 2023. PMID: 37701434 Free PMC article.
-
AAV-mediated gene therapy: Advancing cardiovascular disease treatment.Front Cardiovasc Med. 2022 Aug 19;9:952755. doi: 10.3389/fcvm.2022.952755. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36061546 Free PMC article. Review.
-
Knock-Out of IKKepsilon Ameliorates Atherosclerosis and Fatty Liver Disease by Alterations of Lipid Metabolism in the PCSK9 Model in Mice.Int J Mol Sci. 2024 Oct 5;25(19):10721. doi: 10.3390/ijms251910721. Int J Mol Sci. 2024. PMID: 39409049 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

