Insights into the NAD+ biosynthesis pathways involved during meiotic maturation and spindle formation in porcine oocytes
- PMID: 35342119
- PMCID: PMC9184828
- DOI: 10.1262/jrd.2021-130
Insights into the NAD+ biosynthesis pathways involved during meiotic maturation and spindle formation in porcine oocytes
Abstract
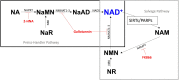
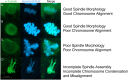
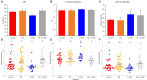
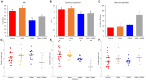
Treatments that elevate NAD+ levels have been found to improve oocyte quality in mice, cattle, and pigs, suggesting that NAD+ is vital during oocyte maturation. This study aimed to examine the influence of different NAD+ biosynthetic pathways on oocyte quality by inhibiting key enzymes. Porcine oocytes from small antral follicles were matured for 44 h in a defined maturation system supplemented with 2-hydroxynicotinic acid [2-HNA, nicotinic acid phosphoribosyltransferase (NAPRT) inhibitor], FK866 [nicotinamide phosphoribosyltransferase (NAMPT) inhibitor], or gallotannin [nicotinamide mononucleotide adenylyltransferase (NMNAT) inhibitor] and their respective NAD+ pathway modulators (nicotinic acid, nicotinamide, and nicotinamide mononucleotide, respectively). Cumulus expansion was assessed after 22 h of maturation. At 44 h, maturation rates were determined and mature oocytes were fixed and stained to assess spindle formation. Each enzyme inhibitor reduced oocyte maturation rate and adversely affected spindle formation, indicating that NAD+ is required for meiotic spindle assembly. Furthermore, NAMPT and NMNAT inhibition reduced cumulus expansion, whereas NAPRT inhibition affected chromosomal segregation. Treating oocytes with gallotannin and nicotinamide mononucleotide together showed improvements in spindle width, while treating oocytes with 2-HNA and nicotinic acid combined showed an improvement in both spindle length and width. These results indicate that the salvage pathway plays a vital role in promoting oocyte meiotic progression, while the Preiss-Handler pathway is essential for spindle assembly.
Keywords: In vitro maturation; Meiotic spindle; NAD+ precursors; Oocyte; Pig.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Supplementing media with NAD+ precursors enhances the in vitro maturation of porcine oocytes.J Reprod Dev. 2021 Oct 29;67(5):319-326. doi: 10.1262/jrd.2021-080. Epub 2021 Aug 19. J Reprod Dev. 2021. PMID: 34408103 Free PMC article.
-
NAMPT regulates mitochondria function and lipid metabolism during porcine oocyte maturation.J Cell Physiol. 2024 Jan;239(1):180-192. doi: 10.1002/jcp.31156. Epub 2023 Nov 22. J Cell Physiol. 2024. PMID: 37992208
-
NAMPT reduction-induced NAD+ insufficiency contributes to the compromised oocyte quality from obese mice.Aging Cell. 2021 Nov;20(11):e13496. doi: 10.1111/acel.13496. Epub 2021 Oct 18. Aging Cell. 2021. PMID: 34662475 Free PMC article.
-
Inhibitors of NAD+ Production in Cancer Treatment: State of the Art and Perspectives.Int J Mol Sci. 2024 Feb 8;25(4):2092. doi: 10.3390/ijms25042092. Int J Mol Sci. 2024. PMID: 38396769 Free PMC article. Review.
-
Regulation of cumulus expansion and hyaluronan synthesis in porcine oocyte-cumulus complexes during in vitro maturation.Endocr Regul. 2012 Oct;46(4):225-35. doi: 10.4149/endo_2012_04_225. Endocr Regul. 2012. PMID: 23127506 Review.
Cited by
-
Can Nicotinamide Adenine Dinucleotide (NAD+) and Sirtuins Be Harnessed to Improve Mare Fertility?Animals (Basel). 2024 Jan 7;14(2):193. doi: 10.3390/ani14020193. Animals (Basel). 2024. PMID: 38254361 Free PMC article. Review.
-
Beyond Pellagra-Research Models and Strategies Addressing the Enduring Clinical Relevance of NAD Deficiency in Aging and Disease.Cells. 2023 Feb 3;12(3):500. doi: 10.3390/cells12030500. Cells. 2023. PMID: 36766842 Free PMC article. Review.
-
Supplemental Nicotinic Acid Elevates NAD+ Precursors in the Follicular Fluid of Mares.Animals (Basel). 2022 May 27;12(11):1383. doi: 10.3390/ani12111383. Animals (Basel). 2022. PMID: 35681847 Free PMC article.
-
NAD+, Sirtuins and PARPs: enhancing oocyte developmental competence.J Reprod Dev. 2022 Dec 19;68(6):345-354. doi: 10.1262/jrd.2022-052. Epub 2022 Sep 27. J Reprod Dev. 2022. PMID: 36171094 Free PMC article. Review.
-
Reproductive Ageing: Metabolic contribution to age-related chromosome missegregation in mammalian oocytes.Reproduction. 2024 Jun 28;168(2):e230510. doi: 10.1530/REP-23-0510. Print 2024 Aug 1. Reproduction. 2024. PMID: 38718822 Free PMC article. Review.
References
-
- Albertini DF, Wickramasinghe D, Messinger S, Mattson BA, Plancha CE. Nuclear and cytoplasmic changes during oocyte maturation. In: Bavister BD (ed.), Preimplantation Embryo Development. Springer-Verlag, New York, 1993; 3–21.
-
- Carneiro GF, Liu IKM, Hyde D, Anderson GB, Lorenzo PL, Ball BA. Quantification and distribution of equine oocyte cortical granules during meiotic maturation and after activation. Mol Reprod Dev 2002; 63: 451–458. - PubMed
-
- Hinrichs K. The equine oocyte: factors affecting meiotic and developmental competence. Mol Reprod Dev 2010; 77: 651–661. - PubMed
-
- Mohammadi-Sangcheshmeh A, Held E, Ghanem N, Rings F, Salilew-Wondim D, Tesfaye D, Sieme H, Schellander K, Hoelker M. G6PDH-activity in equine oocytes correlates with morphology, expression of candidate genes for viability, and preimplantative in vitro development. Theriogenology 2011; 76: 1215–1226. - PubMed
-
- Soifer D. Factors regulating the presence of microtubules in cells. Ann N Y Acad Sci 1986; 466: 1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

