SARS-CoV-2-Legionella Co-Infections: A Systematic Review and Meta-Analysis (2020-2021)
- PMID: 35336074
- PMCID: PMC8951730
- DOI: 10.3390/microorganisms10030499
SARS-CoV-2-Legionella Co-Infections: A Systematic Review and Meta-Analysis (2020-2021)
Abstract
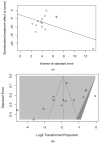
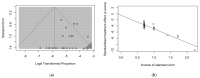
Legionnaires' Disease (LD) is a severe, sometimes fatal interstitial pneumonia due to Legionella pneumophila. Since the inception of the SARS-CoV-2 pandemic, some contradictory reports about the effects of lockdown measures on its epidemiology have been published, but no summary evidence has been collected to date. Therefore, we searched two different databases (PubMed and EMBASE) focusing on studies that reported the occurrence of LD among SARS-CoV-2 cases. Data were extracted using a standardized assessment form, and the results of such analyses were systematically reported, summarized, and compared. We identified a total of 38 articles, including 27 observational studies (either prospective or retrospective ones), 10 case reports, and 1 case series. Overall, data on 10,936 SARS-CoV-2 cases were included in the analyses. Of them, 5035 (46.0%) were tested for Legionella either through urinary antigen test or PCR, with 18 positive cases (0.4%). A pooled prevalence of 0.288% (95% Confidence Interval (95% CI) 0.129-0.641), was eventually calculated. Moreover, detailed data on 19 co-infections LD + SARS-CoV-2 were obtained (males: 84.2%; mean age: 61.9 years, range 35 to 83; 78.9% with 1 or more underlying comorbidities), including 16 (84.2%) admissions to the ICU, with a Case Fatality Ratio of 26.3%. In summary, our analyses suggest that the occurrence of SARS-CoV-2-Legionella infections may represent a relatively rare but not irrelevant event, and incident cases are characterized by a dismal prognosis.
Keywords: COVID-19; Legionella pneumophila typing; Legionnaires’ disease; SARS-CoV-2; diagnosis; epidemiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Fatal Co-infections with SARS-CoV-2 and Legionella pneumophila, England.Emerg Infect Dis. 2021 Nov;27(11):2950-2952. doi: 10.3201/eid2711.204121. Emerg Infect Dis. 2021. PMID: 34670660 Free PMC article.
-
Atypical bacterial co-infections among patients with COVID-19: A study from India.J Med Virol. 2022 Jan;94(1):303-309. doi: 10.1002/jmv.27324. Epub 2021 Sep 15. J Med Virol. 2022. PMID: 34491594 Free PMC article.
-
Co-infection with Legionella and SARS-CoV-2, France, March 2020.Emerg Infect Dis. 2021 Nov;27(11):2864-2868. doi: 10.3201/eid2711.202150. Epub 2021 Sep 1. Emerg Infect Dis. 2021. PMID: 34469708 Free PMC article.
-
Slowly or Nonresolving Legionnaires' Disease: Case Series and Literature Review.Clin Infect Dis. 2020 Apr 15;70(9):1933-1940. doi: 10.1093/cid/ciz538. Clin Infect Dis. 2020. PMID: 31242293 Review.
-
[Legionnaires' disease with pronounced cerebellar involvement: case report and literature review].Zhonghua Jie He He Hu Xi Za Zhi. 2020 Feb 12;43(2):126-131. doi: 10.3760/cma.j.issn.1001-0939.2020.02.010. Zhonghua Jie He He Hu Xi Za Zhi. 2020. PMID: 32062882 Review. Chinese.
Cited by
-
Legionella pneumophila Cas2 Promotes the Expression of Small Heat Shock Protein C2 That Is Required for Thermal Tolerance and Optimal Intracellular Infection.Infect Immun. 2022 Oct 20;90(10):e0036922. doi: 10.1128/iai.00369-22. Epub 2022 Sep 8. Infect Immun. 2022. PMID: 36073935 Free PMC article.
-
Risk Factors of Severe COVID-19: A Review of Host, Viral and Environmental Factors.Viruses. 2023 Jan 7;15(1):175. doi: 10.3390/v15010175. Viruses. 2023. PMID: 36680215 Free PMC article. Review.
-
Severe Legionnaires' disease.Ann Intensive Care. 2024 Apr 2;14(1):51. doi: 10.1186/s13613-024-01252-y. Ann Intensive Care. 2024. PMID: 38565811 Free PMC article. Review.
-
False-positive Legionella pneumophila antibodies in COVID-19 patients.Intensive Care Med Exp. 2023 May 26;11(1):29. doi: 10.1186/s40635-023-00512-8. Intensive Care Med Exp. 2023. PMID: 37231291 Free PMC article. No abstract available.
-
Legionellosis-Associated Hospitalization in Spain from 2002 to 2021.Microorganisms. 2023 Jun 29;11(7):1693. doi: 10.3390/microorganisms11071693. Microorganisms. 2023. PMID: 37512866 Free PMC article.
References
-
- Ullrich A., Schranz M., Rexroth U., Hamouda O., Schaade L., Diercke M., Boender T.S. Impact of the COVID-19 pandemic and associated non-pharmaceutical interventions on other notifiable infectious diseases in Germany: An analysis of National Surveillance Data during Week 1—2016–Week 32—2020. Lancet Reg. Health—Eur. 2021;6:100103. doi: 10.1016/j.lanepe.2021.100103. - DOI - PMC - PubMed
-
- Oh D.-Y., Buda S., Biere B., Reiche J., Schlosser F., Duwe S., Wedde M., von Kleist M., Mielke M., Wolff T., et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January–September 2020: Analysis of National Surveillance Data. Lancet Reg. Health—Eur. 2021;6:100112. doi: 10.1016/j.lanepe.2021.100112. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

