Neuroprotective Effects of Nicotinamide (Vitamin B3) on Neurodegeneration in Diabetic Rat Retinas
- PMID: 35334819
- PMCID: PMC8950738
- DOI: 10.3390/nu14061162
Neuroprotective Effects of Nicotinamide (Vitamin B3) on Neurodegeneration in Diabetic Rat Retinas
Abstract
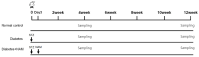
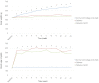
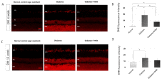
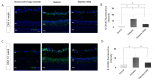
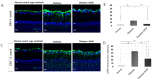
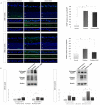
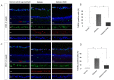
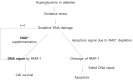
The loss of inner retinal neurons is an initial event in diabetic retinopathy. In diabetic retinas, oxidative stress is increased, which could lead to increased oxidative DNA damage. Nicotinamide is a precursor to nicotinamide adenine dinucleotide, which contributes to the DNA damage response. We investigated whether nicotinamide plays a neuroprotective role in diabetic retinal neurodegeneration in terms of DNA repair. Male Sprague Dawley rats with streptozotocin-induced diabetes were orally administered nicotinamide (500 mg/kg/day) for 4 or 12 weeks. Oxidative stress exhibited by dihydroethidium was upregulated at 4 and 12 weeks after onset of diabetes, and nicotinamide treatment reduced oxidative stress at 4 weeks after induction of diabetes. Oxidative DNA damage measured by 8-hydroxy-2'-deoxyguanosine (8-OHdG) increased at 4 and 12 weeks after induction of diabetes and decreased following nicotinamide treatment. The elevated expression of glial fibrillary acidic protein (GFAP) induced by diabetes was attenuated by nicotinamide treatment. In Western blot analysis, the increased expression of cleaved PARP-1 in diabetes was attenuated by nicotinamide treatment at 12 weeks after induction of diabetes. The diabetes-induced apoptosis of inner retinal cells detected by the TUNEL assay was reduced by nicotinamide treatment. In conclusion, nicotinamide attenuated retinal neurodegeneration in diabetes, probably by reducing oxidative DNA damage and supporting DNA repair.
Keywords: DNA repair; apoptosis; diabetic retinopathy; neuroprotection; nicotinamide; retinal ganglion cell.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Exploring Neuroprotective Effects of Topical Brimonidine in Experimental Diabetic Retinopathy.In Vivo. 2024 Jul-Aug;38(4):1609-1620. doi: 10.21873/invivo.13611. In Vivo. 2024. PMID: 38936912 Free PMC article.
-
Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats.Exp Eye Res. 2014 Aug;125:193-202. doi: 10.1016/j.exer.2014.06.009. Epub 2014 Jun 18. Exp Eye Res. 2014. PMID: 24952278
-
Nitrosative Stress in the Rat Retina at the Onset of Streptozotocin-Induced Diabetes.Cell Physiol Biochem. 2017;42(6):2353-2363. doi: 10.1159/000480007. Epub 2017 Aug 18. Cell Physiol Biochem. 2017. PMID: 28848155
-
Diabetic Retinopathy: Role of Neurodegeneration and Therapeutic Perspectives.Asia Pac J Ophthalmol (Phila). 2022 Mar-Apr 01;11(2):160-167. doi: 10.1097/APO.0000000000000510. Asia Pac J Ophthalmol (Phila). 2022. PMID: 35533335 Review.
-
Meat Intake and the Dose of Vitamin B3 - Nicotinamide: Cause of the Causes of Disease Transitions, Health Divides, and Health Futures?Int J Tryptophan Res. 2017 May 3;10:1178646917704662. doi: 10.1177/1178646917704662. eCollection 2017. Int J Tryptophan Res. 2017. PMID: 28579801 Free PMC article. Review.
Cited by
-
Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration.Pharmaceuticals (Basel). 2024 Jun 26;17(7):837. doi: 10.3390/ph17070837. Pharmaceuticals (Basel). 2024. PMID: 39065688 Free PMC article. Review.
-
A Blended Vitamin Supplement Improves Spatial Cognitive and Short-Term Memory in Aged Mice.Int J Mol Sci. 2024 Feb 28;25(5):2804. doi: 10.3390/ijms25052804. Int J Mol Sci. 2024. PMID: 38474050 Free PMC article.
-
Mitochondria in Retinal Ganglion Cells: Unraveling the Metabolic Nexus and Oxidative Stress.Int J Mol Sci. 2024 Aug 7;25(16):8626. doi: 10.3390/ijms25168626. Int J Mol Sci. 2024. PMID: 39201313 Free PMC article. Review.
-
Exploring Neuroprotective Effects of Topical Brimonidine in Experimental Diabetic Retinopathy.In Vivo. 2024 Jul-Aug;38(4):1609-1620. doi: 10.21873/invivo.13611. In Vivo. 2024. PMID: 38936912 Free PMC article.
-
Poly (ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors in Diabetic Retinopathy: An Attractive but Elusive Choice for Drug Development.Pharmaceutics. 2024 Oct 11;16(10):1320. doi: 10.3390/pharmaceutics16101320. Pharmaceutics. 2024. PMID: 39458649 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

