The regulation of cardiac intermediary metabolism by NADPH oxidases
- PMID: 35325070
- PMCID: PMC9847558
- DOI: 10.1093/cvr/cvac030
The regulation of cardiac intermediary metabolism by NADPH oxidases
Abstract
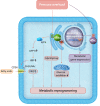
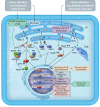
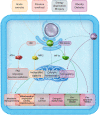
NADPH oxidases (NOXs), enzymes whose primary function is to generate reactive oxygen species, are important regulators of the heart's physiological function and response to pathological insults. The role of NOX-driven redox signalling in pathophysiological myocardial remodelling, including processes such as interstitial fibrosis, contractile dysfunction, cellular hypertrophy, and cell survival, is well recognized. While the NOX2 isoform promotes many detrimental effects, the NOX4 isoform has attracted considerable attention as a driver of adaptive stress responses both during pathology and under physiological states such as exercise. Recent studies have begun to define some of the NOX4-modulated mechanisms that may underlie these adaptive responses. In particular, novel functions of NOX4 in driving cellular metabolic changes have emerged. Alterations in cellular metabolism are a recognized hallmark of the heart's response to physiological and pathological stresses. In this review, we highlight the emerging roles of NOX enzymes as important modulators of cellular intermediary metabolism in the heart, linking stress responses not only to myocardial energetics but also other functions. The novel interplay of NOX-modulated redox signalling pathways and intermediary metabolism in the heart is unravelling a new aspect of the fascinating biology of these enzymes which will inform a better understanding of how they drive adaptive responses. We also discuss the implications of these new findings for therapeutic approaches that target metabolism in cardiac disease.
Keywords: Cardiac metabolism; Intermediary metabolism; NADPH oxidases; Redox signalling.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.M.S. serves as an adviser to Forcefield Therapeutics and CYTE—Global Network for Clinical Research. This manuscript was handled by Reviews Deputy Editor Dr Ali J. Marian. None of the other authors declare any conflict of interest.
Figures


Similar articles
-
Nicotinamide Adenosine Dinucleotide Phosphate Oxidase-Mediated Signaling in Cardiac Remodeling.Antioxid Redox Signal. 2023 Feb;38(4-6):371-387. doi: 10.1089/ars.2022.0176. Antioxid Redox Signal. 2023. PMID: 36656669 Review.
-
NADPH oxidases and cardiac remodelling.Heart Fail Rev. 2011 Jan;16(1):5-12. doi: 10.1007/s10741-010-9186-2. Heart Fail Rev. 2011. PMID: 20658317 Review.
-
Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury.J Am Heart Assoc. 2014 Jan 27;3(1):e000555. doi: 10.1161/JAHA.113.000555. J Am Heart Assoc. 2014. PMID: 24470522 Free PMC article.
-
NOX Dependent ROS Generation and Cell Metabolism.Int J Mol Sci. 2023 Jan 20;24(3):2086. doi: 10.3390/ijms24032086. Int J Mol Sci. 2023. PMID: 36768405 Free PMC article. Review.
-
NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart.Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15565-70. doi: 10.1073/pnas.1002178107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713697 Free PMC article.
Cited by
-
KLB and NOX4 expression levels as potential blood-based transcriptional biomarkers of physical activity in children.Sci Rep. 2023 Apr 5;13(1):5563. doi: 10.1038/s41598-023-31537-4. Sci Rep. 2023. PMID: 37019912 Free PMC article.
-
Oxidative Stress, Antioxidants and Hypertension.Antioxidants (Basel). 2023 Jan 27;12(2):281. doi: 10.3390/antiox12020281. Antioxidants (Basel). 2023. PMID: 36829839 Free PMC article. Review.
-
Health position paper and redox perspectives on reactive oxygen species as signals and targets of cardioprotection.Redox Biol. 2023 Nov;67:102894. doi: 10.1016/j.redox.2023.102894. Epub 2023 Oct 6. Redox Biol. 2023. PMID: 37839355 Free PMC article. Review.
-
MYH7 R453C induced cardiac remodelling via activating TGF-β/Smad2/3, ERK1/2 and Nox4/ROS/NF-κB signalling pathways.Open Biol. 2024 Jun;14(6):230427. doi: 10.1098/rsob.230427. Epub 2024 Jun 12. Open Biol. 2024. PMID: 38862020 Free PMC article.
-
Influence of Isolated Resistance Exercise on Cardiac Remodeling, Myocardial Oxidative Stress, and Metabolism in Infarcted Rats.Antioxidants (Basel). 2023 Apr 7;12(4):896. doi: 10.3390/antiox12040896. Antioxidants (Basel). 2023. PMID: 37107271 Free PMC article.
References
-
- Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007;356:1140–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

