Engineering Novel Lentiviral Vectors for Labelling Tumour Cells and Oncogenic Proteins
- PMID: 35324780
- PMCID: PMC8945451
- DOI: 10.3390/bioengineering9030091
Engineering Novel Lentiviral Vectors for Labelling Tumour Cells and Oncogenic Proteins
Abstract
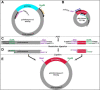
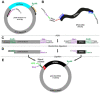
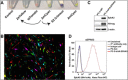
Lentiviral vectors are unique and highly efficient genetic tools to incorporate genetic materials into the genome of a variety of cells whilst conserving biosafety. Their rapid acceptance made it necessary to improve existing protocols, including molecular engineering and cloning, production of purified lentiviral particles, and efficient infection of target cells. In addition to traditional protocols, which can be time-consuming, several biotechnology companies are providing scientists with commercially available lentiviral constructs and particles. However, these constructs are limited by their original form, tend to be costly, and lack the flexibility to re-engineer based on the ever-changing needs of scientific projects. Therefore, the current study organizes the existing methods and integrates them with novel ideas to establish a protocol that is simple and efficient to implement. In this study we, (i) generated an innovative site-directed nucleotide attachment/replacement and DNA insertion method using unique PCR primers, (ii) improved traditional methods by integrating plasmid clarification steps, (iii) utilized endogenous mRNA as a resource to construct new lentiviruses, and (iv) identified an existing purification method and incorporated it into an organized workflow to produce high-yield lentiviral particle collection. Finally, (v) we verified and demonstrated the functional validity of our methods using an infection strategy.
Keywords: genetic engineering; lentiviral particle; lentivirus; molecular cloning; plasmid; site-directed mutagenesis; tumour heterogeneity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Production of lentiviral vectors for transducing cells from the central nervous system.J Vis Exp. 2012 May 24;(63):e4031. doi: 10.3791/4031. J Vis Exp. 2012. PMID: 22664962 Free PMC article.
-
Detailed design and comparative analysis of protocols for optimized production of high-performance HIV-1-derived lentiviral particles.Metab Eng. 2005 Sep-Nov;7(5-6):426-36. doi: 10.1016/j.ymben.2005.06.006. Epub 2005 Aug 15. Metab Eng. 2005. PMID: 16102993
-
Packaging HIV- or FIV-based lentivector expression constructs and transduction of VSV-G pseudotyped viral particles.J Vis Exp. 2012 Apr 8;(62):e3171. doi: 10.3791/3171. J Vis Exp. 2012. PMID: 22508377 Free PMC article.
-
Non-integrating lentiviral vectors.Curr Gene Ther. 2008 Dec;8(6):430-7. doi: 10.2174/156652308786848012. Curr Gene Ther. 2008. PMID: 19075626 Review.
-
Lentiviral Delivery of Proteins for Genome Engineering.Curr Gene Ther. 2016;16(3):194-206. doi: 10.2174/1566523216666160527143702. Curr Gene Ther. 2016. PMID: 27228988 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

